Advancements in food allergen immunotherapy: improving quality of life and reducing risks
Article information
Key message
· Pediatric food allergies considerably impair patient and family quality of life, particularly those with persistent allergies to common food allergens.
· Recent research has focused on developing diverse approaches to food allergen immunotherapy, showing promising outcomes of oral, sublingual, and epicutaneous immuno therapies.
· Critical considerations in immunotherapy candidate selection underscore the need for personalized approaches and reliable biomarkers in future studies to improve treatment outcomes.
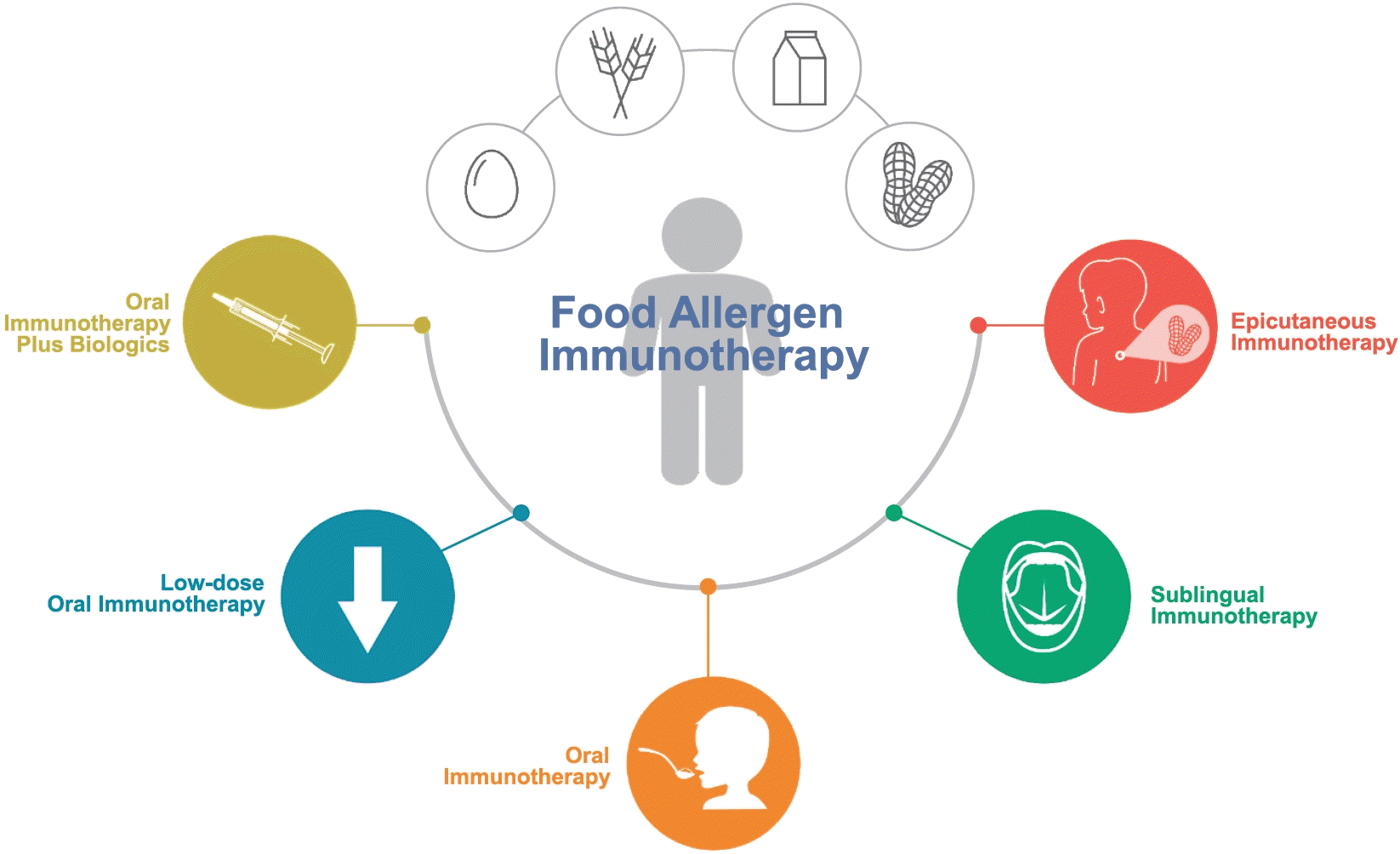
Graphical abstract.
The prevalence of food allergies (FAs) has increased in recent decades, affecting 5%–10% of children worldwide [1,2]. FA has emerged as a growing concern due to the substantial risks and reduced quality of life (QoL) of patients and their families [3,4]. Of note, in approximately 50% of Korean children allergic to eggs or milk, these allergies persist to the ages of 5 and 8 years, respectively [5]. Moreover, parents of patients with FA aged ≥5 years show significant concern about their children's vigilance while eating at school and engaging in preschool/school activities involving food [4]. Taken together, interventions for patients with FA, such as FA immunotherapy, can potentially improve the prognosis of children with FA and the QoL of them and their family members.
Although FA management for the past several decades has focused on allergen avoidance, recent research advancements have facilitated diverse approaches to FA immunotherapy [6]. Burks et al. conducted a double-blind randomized placebo-controlled trial and long-term follow-up of the effectiveness and safety of oral immunotherapy (OIT) for children with egg allergies [7]. This study revealed that 55% of children in the OIT group versus 0% in the control group demonstrated desensitization after 10 months of OIT [7]. Additionally, 28% of children in the OIT group passed the oral food challenge after 22 months of OIT and 4–6 weeks of avoiding egg consumption, indicating sustained unresponsiveness. Since the introduction of OIT for FA, its application has expanded, and delivery methods have been extensively studied [6,8-9]. For example, a recent study compared peanut sublingual immunotherapy (SLIT) with placebo in 40 adolescents and adults with peanut allergies [9]. This study demonstrated a significant increase in the median dose that was successfully consumed after 44 weeks of SLIT versus no increase in the placebo group. Epicutaneous immunotherapy (EPIT), which involves the application of a FA via a skin patch, was examined in a phase 3, multicenter, double-blind, randomized, placebo-controlled trial of patients aged 1–3 years [10]. The results indicated desensitization in 67.0% of children in the EPIT group versus 33.5% of children in the placebo group (P<0.001) after 12 months of treatment.
In this issue of Clinical and Experimental Pediatrics, Jeon and Kim [6] offer a comprehensive summary of recent studies of various current FA immunotherapy strategies with focus on their respective advantages and disadvantages. This article provides detailed information on study populations, allergen dosage, treatment duration, efficacy, and safety of OIT, SLIT, and EPIT. Although OIT has known ability to induce desensitization and improve disease prognosis among children with FAs, safety concerns pose significant barriers to its widespread application. Adverse events ranging from mild symptoms to potentially life-threatening reactions have been documented in 30%–100% of patients undergoing OIT [6]. Therefore, this article emphasizes the therapeutic potential of promising strategies such as low-dose immunotherapy and combination therapies with biologics including omalizumab, dupilumab, and ligelizumab. For example, a recent real-world study of 58 children with severe milk allergies treated with omalizumab revealed that 83% tolerated ≥6,000 mg of milk protein during the maintenance phase [8]. In contrast, only 40.5% of those treated with OIT without omalizumab could tolerate milk. Adverse events were noted in 59% of patients who discontinued omalizumab compared to 25% of those who continued treatment. Moreover, patients who suddenly discontinued treatment experienced more adverse events than those who gradually discontinued treatment, although the difference was not statistically significant. These various approaches aim to minimize allergic reactions and enhance treatment efficacy among patients with FAs.
The study of Jeon and Kim [6] underscores critical considerations in selecting candidates for FA immunotherapy by emphasizing various factors such as causative allergen, potential for natural outgrowing, symptom severity, and patient age. Notably, children facing multiple food restrictions or allergies to common allergens such as eggs, milk, soy, or wheat tend to experience more compromised QoL than those with single or uncommon allergens [4]. Moreover, parents of children with a history of food-induced anaphylaxis bear a significant burden, with over half of mothers experiencing posttraumatic stress symptoms [3,4]. Personalized FA immunotherapy plans play a pivotal role in identifying the most suitable treatment approach for each patient, contributing to improving the QoL of individuals with FAs. Therefore, future studies should focus on developing tailored methods that align with the treatment goals as well as identifying reliable biomarkers to predict which candidates are more likely to achieve desensitization and tolerance. These insights hold promise for advancing treatment approaches and further individualizing patient care.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (2023R1A2C1002740).
Acknowledgements
The authors thank Bo-Hyeon Kim and Young-Hee Chung from Multimedia Services Part, Samsung Medical Center (Seoul, Korea) for drafting the graphical abstract.