Double-negative T cells in pediatric rheumatic diseases
Article information
Abstract
Double-negative (CD4-CD8-) T (DNT) cells have been implicated in autoimmune lymphoproliferative syndrome (ALPS), where their expansion inside the circulating pool of T cells represents a diagnostic criterion. Recent experimental evidence has supported the immunomodulatory roles of DNT cells, and studies in adult patients have suggested that they may be altered in some immune-mediated conditions. This study aimed to retrieve available data on circulating DNT cells in pediatric rheumatic disorders that do not arise in the context of ALPS through a systematic literature review of 3 scientific databases (PubMed, Scopus, and Web of Science). The final output of the systematic literature search consisted of 8 manuscripts, including cross-sectional (n=6) and longitudinal (n=2) studies. Overall, the pooled population of patients includes children affected with pediatric systemic lupus erythematosus (n= 104), juvenile idiopathic arthritis (n=92), Behçet's disease (n=15), mixed connective tissue disease (n=8), juvenile dermatomyositis (n=6), and Kawasaki disease/multisystem inflammatory disease in children (n=1 and n=14, respectively); moreover, one study also included 11 children with a high titer of antinuclear antibody but no diagnosis of rheumatic disease. All studies except one included a control group. The number of DNT cells were increased in most studies of children with rheumatic diseases. Even if such a limited number of studies and their great heterogeneity in several methodological aspects do not allow for reliable conclusions about the relevance of DNT cells in specific rheumatic conditions in children, this cell population deserves further investigation in this pathological setting through well-designed clinical studies.
Key message
Double-negative T (DNT) cells appear to be increased in several pediatric rheumatic diseases and this finding may be correlated with disease activity to some extent. However, due to significant heterogeneity in several methodological aspects, further investigations in rheumatic children are needed to assess the potential relevance of DNT cells as biomarkers and clarify their immunopathological role.
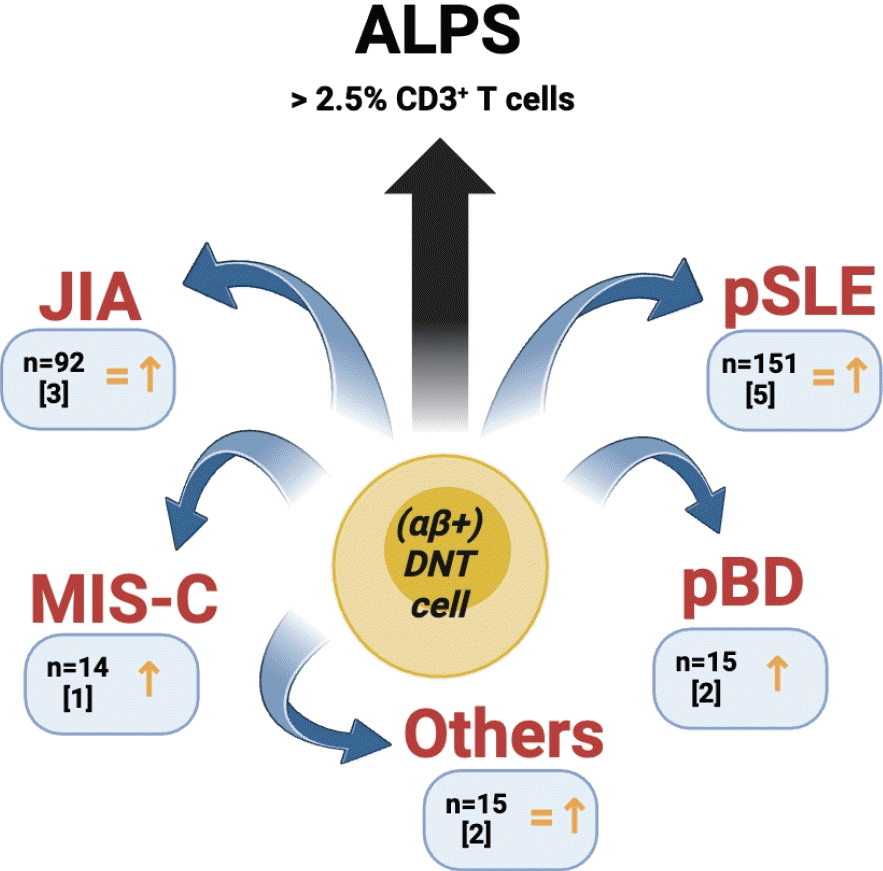
Graphical abstract. DNT cells are well known to be increased in ALPS, but scarce information is available for pediatric rheumatic diseases in which DNT cell numbers are variably but inconsistently increased, according to the present systematic review (“n” indicates the number of pooled patients from the studies selected in this systematic review; the number between square brackets indicates the number of studies including specific rheumatic children; the symbols below each disease indicatively represent the findings related to circulating DNT cells in the corresponding studies). ALPS, autoimmune lymphoproliferative syndrome; DNT, double-negative T cells; JIA, juvenile idiopathic arthritis; MIS-C, multisystem inflammatory syndrome in children; pBD, pediatric Behçet's disease; pSLE, pediatric systemic lupus erythematosus.
Introduction
The term “double-negative T (DNT) cells” is currently used to indicate an unconventional T-cell subset, which is CD3+, but expresses neither CD4/CD8 molecules nor natural killer cells markers. DNT cells are found in both TCRαβ+ and TCRγδ+ T-cell populations; however, the latter are mostly CD4-CD8- cells [1], and the category “DNT cells” is often used to specifically refer to TCRαβ+CD4-CD8- T cells [1,2].
TCRαβ+ cells are the majority of the T-cell pool circulating in the blood: indeed, TCRγδ+ T cells vary between 3% and 10% of peripheral blood T cells in adults and may significantly increase in several lymphoproliferative conditions [3,4].
The most defined pathological setting with expansion of (TCRαβ+) DNT cells is represented by the autoimmune lymphoproliferative syndrome (ALPS), wherein these cells account for >1.5% of total lymphocytes and/or >2.5% of CD3+ lymphocytes (regardless of normal/elevated total lymphocyte count). These conditions constitute a criterion required to diagnose ALPS according to current guidelines [1,5].
Although the precise ontogeny of human DNT cells remains unclear and poorly understood, they could be derived from both thymic and peripheral cells, suggesting the existence of several origins and differentiation pathways [6]. Moreover, DNT cells can display different phenotypical and functional aspects; according to some research findings from murine models, they may be divided into inflammatory and regulatory subpopulations, the former of which have pro-inflammatory and cytolytic properties (based on the production of interferon-γ, tumor necrosis factor-α, and granzyme), whereas the latter can exert suppressive activities through interleukin (IL)-10 and Fas-Fas ligand pathway, especially on activated CD4+ and CD8+ T cells [6-8].
Owing to these recent observations of their inflammatory and regulatory properties emerging from murine models, DNT cells have received attention in immunopathological contexts other than ALPS, including rheumatic disorders, cancer, and infectious diseases [6]. Several human studies have suggested that DNT cells can be expanded in patients diagnosed with systemic lupus erythematosus (SLE) and could represent a major source of IL-17A [9,10], which has also been observed in other adult rheumatic disorders such as psoriasis and Sjögren’s syndrome [11-13].
Here, a systematic literature review analyzed the current scientific evidence regarding potential alterations in the DNT cell population in pediatric rheumatic disorders.
Methods
1. Protocol
This systematic review of the medical literature included all original articles and case reports/series providing information on DNT cells in children with pediatric rheumatic diseases, except for those finally or concomitantly diagnosed with ALPS. The primary aim of this systematic review was to assess the number and, if available, additional phenotypic characteristics of DNT cells in these patients. This systematic literature search excluded reviews, conference papers, letters, and book chapters.
2. Search strategy
To retrieve all original articles and case reports/series according to the research aims, the systematic literature search was conducted in the PubMed, Scopus, and Web of Science databases (Table 1). The search period was from the database inception through August 31th, 2023.
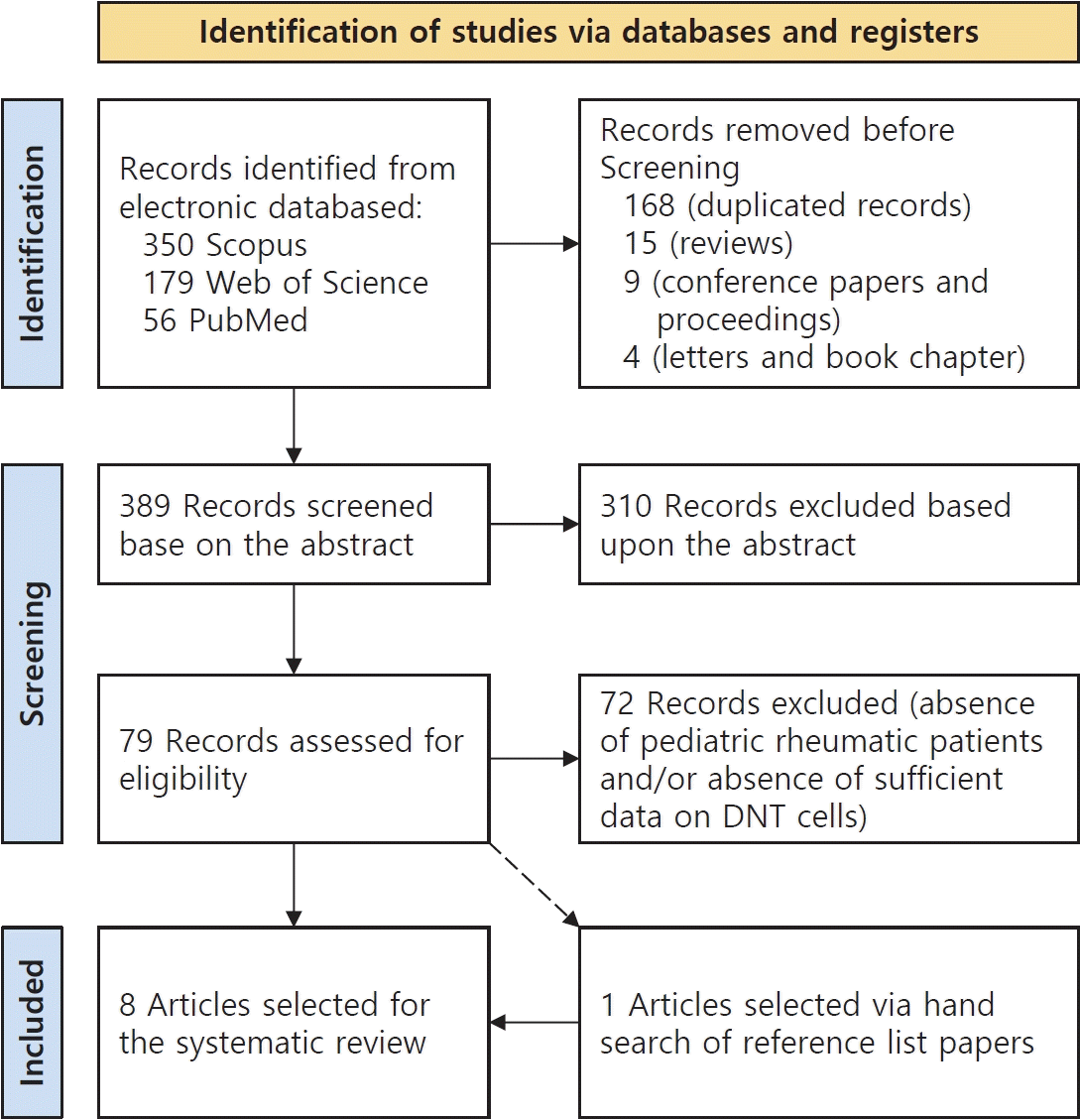
After the retrieval of 585 items from the aforementioned electronic databases, duplicated records (n=168), review articles (n=15), conference papers and proceedings (n=9), and letters and book chapters (n=4) were eliminated; only publications in English and describing human research were considered. Therefore, after this initial screening, 389 titles were screened for eligibility based on the information available in the abstract; of them, 79 papers (case-control, cross-sectional, retrospective studies, case reports, and case series) of children affected with rheumatic diseases and wherein DNT cells have been investigated, were subjected to full-text review. This systematic literature search was performed according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Fig. 1).
3. Data extraction
After a critical assessment of all retrieved articles, the data were extracted by the principal investigator and confirmed by a second investigator following the main inclusion criteria: any original article providing individual or aggregated data on DNT cell number (and, if available, immunophenotypic characteristics) in children with rheumatic disorders without a concomitant ALPS diagnosis. The following items were extracted from each case report/series: first author's last name, publication year, country, study design, study population by rheumatic disease, study participants’ age and sex, study groups, control group (if any), controls’ age and sex, DNT cell immunophenotypic markers, DNT cell numbers, flow cytometry equipment, study participants’ therapy, and DNT cell-related findings.
Results
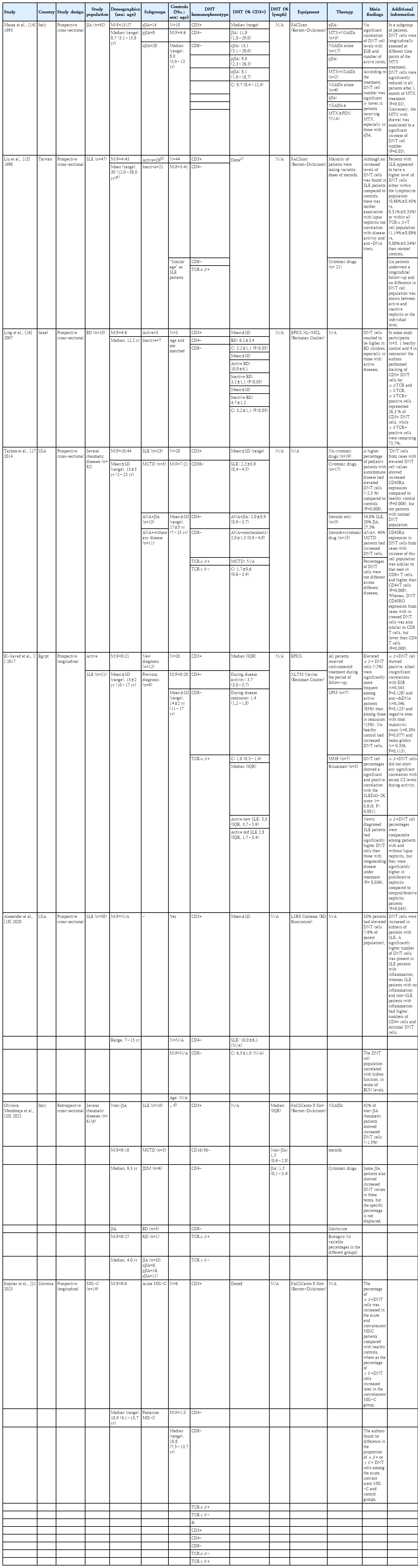
The final output of this systematic literature search consisted of 8 articles, including both cross-sectional (n=6) and longitudinal (n=2) studies. All but one study had prospectively collected their data. The main characteristics and information emerging from these studies are summarized in Table 2 [14-21].
By definition, all of these studies included pediatric patients. However, the study by Liu et al. [15] also included adult patients, who apparently represented the majority of the study population. Indeed, pediatric data were not presented separately. Conversely, all other studies included children only; thus, their aggregated data specifically referred to the pediatric population, although in the study by Tarbox et al. [17], the upper age limit for patient recruitment was set at 21 years.
Based on the aims of this systematic review, children with rheumatic disorders represented the general study population. Different studies enrolled participants affected by several rheumatic diseases, including pediatric SLE (pSLE) (n=5), juvenile idiopathic arthritis (JIA; n=3), Behçet's disease (BD; n=2), mixed connective tissue disease (MCTD; n=2), juvenile dermatomyositis (JDM; n=1), Kawasaki disease (KD; n=1), and multisystem inflammatory syndrome in children (MIS-C; n=1). In fact, 7 studies focused on specific diseases, namely pSLE (n=3), JIA (n=1), BD (n=1), and MIS-C (n=1), whereas 2 studies investigated 2 or more rheumatic disorders.
Overall, the pooled numbers of children affected with different rheumatic diseases were as follows: pSLE (n=104), JIA (n=92), BD (n=15), MCTD (n=8; however, Tarbox et al. [17] did not provide DNT cell number for this rheumatic subgroup due to their very small number), JDM (n=6), and KD/MIS-C (n=1 and n=14, respectively); moreover, as an additional study group (not control), Tarbox et al. [17] included 11 children with a high antinuclear antibody titer (>1:1280) but no diagnosis of rheumatic disease. As already mentioned, the study by Liu et al. [15] included both adult and pediatric patients with SLE, but there was no way to distinguish between them based on the information available from the paper; therefore, these 47 patients with SLE were not included in the above list and count.
All studies except one also included a control group that was variably defined according to the specific study but basically characterized by the absence of a previous or concomitant diagnosis of any autoimmune disorder. This control group was used to compare the DNT cell count for patients with all rheumatic disorders.
In all of these studies, DNT cells were expressed as percentages and never in absolute terms. Most studies calculated DNT cells as the number of T (CD3+) cells (n=5), whereas only one provided this percentage as the number of total lymphocytes. Unfortunately, 2 studies did not provide the numerical values of DNT cells in a table or text; one of them was the aforementioned study by Liu et al. [15], which did not analyze the pediatric data separately.
Almost all patients included in these studies received pharmacological therapy at the time of blood sampling. Aggregated data on ongoing therapy were described in 5 articles, whereas 3 did not provide this information.
Despite the heterogeneity of the study design, populations, aims, and equipment in these clinical studies, most showed an increased percentage of DNT cells in rheumatic children (n=3), or an increased (n=2) or consistent (n=2) number of patients with DNT cell numbers above the reference range. The study by Massa et al. [14] did not highlight any significant differences compared with controls.
In addition to the general measurement of the DNT cell number, 6 studies attempted to assess the association and/or correlation between the DNT cell number and clinical and/or laboratory parameters, 5 of which found one or more significant findings.
Finally, only Tarbox et al. [17] assessed additional surface markers expressed by DNT cells.
Discussion
In this systematic literature search, we summarized the current data on DNT cell populations in pediatric patients with rheumatic disorders.
The first general observation emerging from the present literature analysis is that very few studies (n=8) provided numerical information on circulating DNT cells in this pediatric pathological setting. Of course, this cell population has been extensively studied in children with ALPS, where it has very important diagnostic relevance as explained above [1,5].
In addition to being an important diagnostic marker for ALPS, DNT cells may directly promote the onset of autoimmune and inflammatory phenomena in this disease setting [22]. Indeed, in ALPS (and other diseases) murine models, DNT cells produced remarkable amounts of several inflammatory and immuno-modulatory cytokines, such as IL-2, IL-4, IL-10, tumor necrosis factor-α, and IL-17A [22-24]. Moreover, in ALPS patients (and also murine models), expanded DNT cells showed a number of immuno-phenotypical differences compared to “physiological” DNT cells that can be isolated from healthy individuals [22,25]. Interestingly, some studies indicated that DNT cells promote autoantibody production [9,10]. Finally, some studies, including those of adults affected with SLE, psoriasis, and Sjögren syndrome, have shown that DNT cells can infiltrate their main target organs (kidneys, skin, and salivary glands), suggesting a direct and pathogenic role of these cells in tissue damage [10,11,13,22].
However, despite some evidence implicating a role of DNT cells in different autoimmune phenomena, according to research on experimental models, their homeostasis has been poorly investigated in children with rheumatic disorders. Moreover, the few studies investigating this cell population in children are heterogeneous in several aspects. First, among the 6 studies focusing on individual pediatric rheumatic diseases, 3 investigated SLE [15,18,19] and the remaining 3 referred to one specific disease each, namely JIA, BD, and MIS-C [14,16,21]; notably, one of these 3 SLE studies merged children and adult patients, making it impossible to extract data specific to pSLE [15]. In our literature review, there were 2 additional studies including different rheumatic disorders (JIA, SLE, JDM, MCTD, and KD), whose small numbers per specific disease did not allow any reliable comparison or statistical analysis [14-21].
In addition to the heterogeneous study populations, the immunophenotyping strategies were not identical across the selected studies, and used different FACS equipment (also due to the large time window – 1993/2023 – during which these studies were carried out and published). Even though all the studies expressed DNT cells as percentage of CD3+ cells (except one by Oliveira Mendonça et al. [20], who calculated these cells on the total lymphocytes population), the immunophenotypic definition of DNT cell is not exactly the same in all of them: indeed, 3 studies identified this CD3+ cell population as CD4-CD8- only, 2 studies specified the TCRαβ positivity on these CD4-CD8- population, and the remaining 3 studies also assessed TCRγδ [14-21]. Among the latter ones, Tarbox et al. [17] and Oliveira Mendonça et al. [20] specifically counted CD4-CD8-TCRαβTCRγδ- T cells as DNT population, whereas Kopitar et al. [21] measured both TCRαβ+ and TCRγδ+ CD4-CD8- T cells. Actually, Ling et al. [16] also assessed the expression of TCRαβ and TCRγδ in part of their patients (n=5), although their main results are based only on double negativity of CD8 and CD4 markers, as mentioned above: indeed, through this additional and partial analysis, they only wanted to measure the proportion of CD8-CD4- T cells expressing TCRαβ and TCRγδ (respectively: 26.3% and 73.7%) [16]. Notably, no studies have provided information on the absolute count of DNT cells. Finally, all the studies included patients who received different therapies.
Therefore, such a great study heterogeneity in these methodological aspects, in addition to the different study populations, makes it difficult to compare DNT cell findings across these 8 clinical studies and, then, draw any reliable conclusion, of course. However, several studies have highlighted an increased number of DNT cells in rheumatic diseases or according to some clinical aspects. Liu et al. [15] and Ling et al. [16] observed a higher number of DNT cells in children with SLE and BD, whereas the former study found no correlation with disease activity and/or the presence of nephritis, and the latter study observed a greater increase in DNT, especially in children with active BD [15,16]. Other studies have reported a greater number of patients with DNT counts above the cutoff compared to controls; Tarbox et al. [17] reported such a statistically significant difference by considering all rheumatic study populations (including children with SLE, MCTD, and JIA). El-Sayed et al. [18] found that patients with active pSLE more frequently had increased DNT cell numbers than children with inactive disease, whereas no control had a DNT count above the cutoff value; moreover, they also reported a significant correlation between DNT cell number and disease activity according to Systemic Lupus Erythematosus Disease Activity Index 2000 score. Although the study by Alexander et al. [19] was mainly based on murine experiments, they also included some analysis of human samples; in 53% of their patients with pSLE, they observed elevated DNT cell counts, which also showed some correlation with kidney function.
Similarly, Oliveira Mendonça et al. [20] reported that 42% of patients with non-JIA rheumatic diseases showed increased DNT cell counts, as did some JIA patients (without specifying the exact percentage); however, no statistical comparison was made with any control group since this study mainly included patients with autoinflammatory disorders, which have a different immunopathogenic profile [26,27]. The only available study focused on JIA was authored by Massa et al. [14], who reported no specific difference in DNT cells between these patients and controls; moreover, they found no significant correlation between DNT cell number and ESR or the number of active joints. Finally, the most recent study of our selection investigated patients with MIS-C: Kopitar et al. [21] observed a significant percentage increase of TCRαβ+ DNT cells in patients with MIS-C (acute and postacute) compared to controls; moreover, they also conducted this analysis in TCRγδ+ DNT cells, among which some differences were also noticed, but mainly in the postacute phase [21].
Khojah et al. [28] recently published an abstract in which DNT cells were specifically assessed in patients with JDM. Of course, this scientific contribution was not included in our systematic review because this was not a full article and not all data were available. However, it is worth mentioning and reporting its main preliminary observations, namely that more than 50% of JDM patients showed elevated DNT cell counts despite no correlation with disease activity indexes.
In summary, most studies found some DNT cell-related alterations in different pediatric rheumatic patients and/or according to some clinical features such as specific complications and/or disease activity; however, several aspects greatly limited the possibility of making any clear conclusions about this matter. In addition to the paucity of studies providing information on DNT cells in the pediatric rheumatic setting, the significant heterogeneity (especially in methodological aspects) across available studies, is another important limitation. Indeed, if the available studies are considered overall, different target populations (in terms of specific rheumatic diseases) were included, variable immunophenotyping strategies and flow cytometry methods were used, and the study participants might have been enrolled at various disease stages while receiving different types of treatment.
Although these limitations do not allow any kind of conclusion about the relevance of DNT cells in specific rheumatic conditions in children or, in general, in the rheumatic pathological setting, these preliminary results suggest the need for further attention and investigations on this cell population in children affected with rheumatic diseases, as well as growing evidence on the immunomodulatory role of DNT cells in other clinical situations, such as malignancies, infections, and transplants [6,29-31].
Further pediatric studies focusing on DNT cells in specific rheumatic diseases are needed, and should be standardized in terms of methodological and analytical approaches considering all of the aforementioned limitations highlighted in the available studies. Moreover, future research should include more patients at the initial stage of the rheumatic disease to obtain information on the DNT cells before any therapy may alter their homeostasis. It would be important to perform these studies longitudinally and prospectively to obtain additional information that may be potentially correlated to the clinical response and, in general, the clinical course and prognosis. Finally, the present literature review highlighted the absence of information regarding specific immunophenotypic characteristics in this cell population. Only Tarbox et al. [17] provided information on additional markers such as CD45RA and CD45RO. Therefore, these and other immunophenotypic aspects should be investigated in the future to better define (phenotypic and functional) DNT cell subsets in rheumatic children.
In conclusion, DNT cells were variably but inconsistently increased in most studies investigating children with rheumatic diseases as schematically summarized in the Graphical abstract. Therefore, this cell population deserves more attention and investigations for specific pediatric rheumatic disorders. However, the limited number of studies and their heterogeneity in several methodological aspects prevented us from drawing any clear conclusions or specific hypotheses regarding their relevance as diagnostic markers and/or immunological roles. Further research is needed to assess the relevance of DNT cells in pediatric rheumatic disorders and better define specific DNT cell subsets that may be functionally different and thus potentially serve as biological markers. Prospective and longitudinal studies are needed, which should be based on a standardized methodological strategy to count DNT cells and include patients at diagnosis who have not yet received any specific therapy.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research work was supported by the Nazarbayev University Cooperative Research Grant 2023-2025 (No. 20122022CRP1604); and by the Science Committee of the Ministry of Higher Education and Science of the Republic of Kazakhstan (Grant No. AP19677323).
Author contribution
Conceptualization: DP; Data curation: DP, TM, GZ, KD; Formal analysis: DP, TM, GZ, KD, LA; Funding acquisition: DP; Methodology: DP, TM; Project administration: TM; Visualization: DP, TM, GZ; Writing - original draft: DP; Writing - review & editing:DP, LA. ZM