Polysomnographic features of children with obesity: body mass index predict severe obstructive sleep apnea in obese children?
Article information
Abstract
Background
Few studies have explored the polysomnographic features of children with obesity.
Purpose
This study aimed to explore the demographic and polysomnographic features of obese children and determine whether body mass index (BMI) could predict severe obstructive sleep apnea (OSA).
Methods
This cross-sectional study recruited obese children who underwent diagnostic polysomnography between January 2019 and March 2022. We explored demographic and anthropometric measures as well as polysomnographic abnormalities among them. We used receiver operating characteristic curves and logistic regression analyses to determine the optimal cutoff values of anthropometric variables for predicting severe OSA.
Results
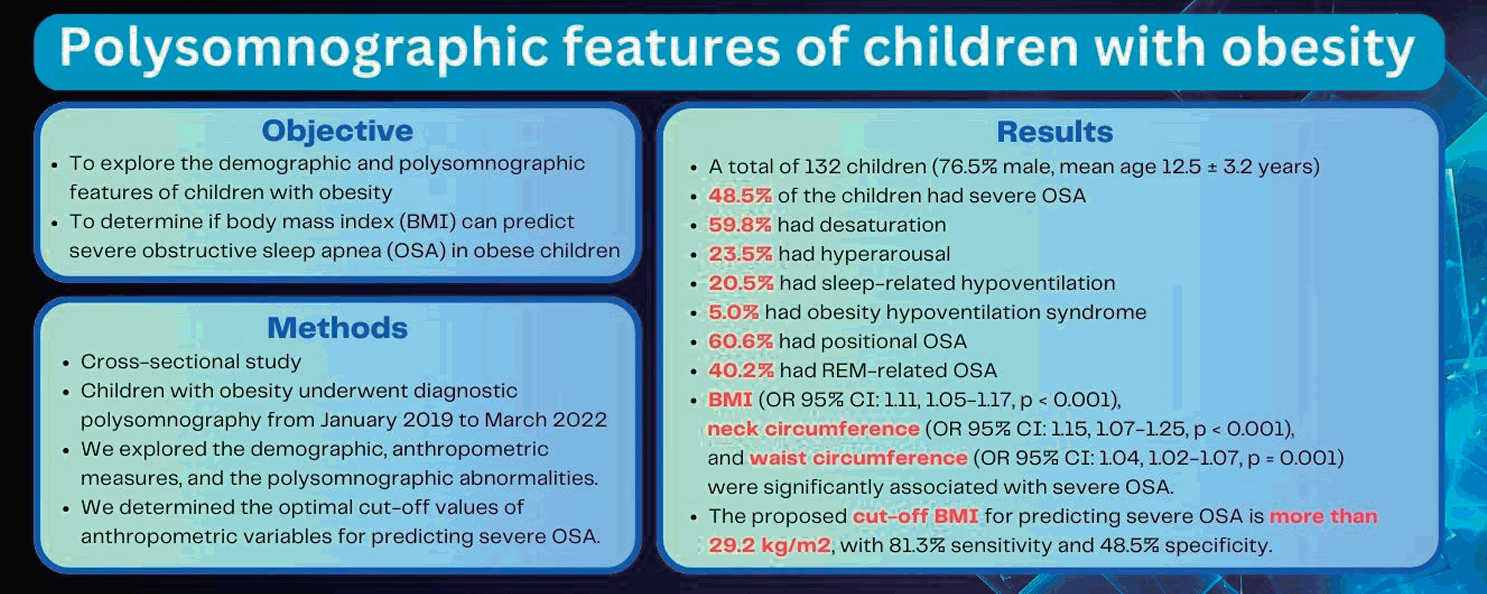
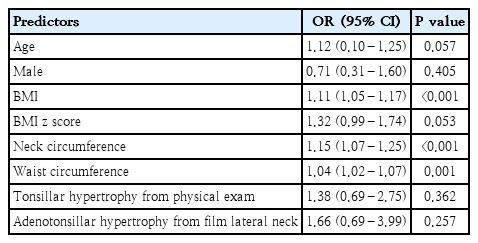
A total of 132 children with obesity (76.5% male; mean age, 12.5±3.2 years) were included. Severe OSA was identified in 64 children (48.5%). Desaturation was observed in 59.8%, while 23.5% had hyperarousal, 20.5% had sleep-related hypoventilation, 60.6% had positional OSA, 40.2% had rapid eye movement-related OSA, and 5.0% had obesity hypoventilation syndrome. Among them, BMI (odds ratio [OR], 1.11; 95% confidence interval [CI], 1.05–1.17; P<0.001), neck circumference (OR, 1.15; 95% CI, 1.07–1.25; P<0.001), and waist circumference (OR, 1.04; 95% CI, 1.02–1.07; P=0.001) were significantly associated with severe OSA. These findings suggest a cutoff BMI for predicting severe OSA of greater than 29.2 kg/m2 with 81.3% sensitivity and 48.5% specificity.
Conclusion
Severe OSA is common in children with obesity; thus, we recommend screening children with obesity and a BMI greater than 29.2 kg/m2 for severe OSA.
Key message
Question: How Common is obstructive sleep apnea (OSA) in obese children? OSA is common in obese children, even without habitual snoring.
Findings: Among the subjects, 60.6% had positional OSA, 40.2% had rapid eye movementrelated OSA, 59.8% had desaturation, 20.5% had sleeprelated hypoventilation, and 5.0% had obesity hypoventilation syndrome. Body mass index (BMI) and neck and waist circumferences were significantly associated with severe OSA.
Meaning: We recommend screening obese children (BMI > 29.2 kg/m2) for OSA.
Graphical abstract. Polysomnographic features of children with obesity. REM, rapid eye movement; OR, odds ratio; CI, confidence interval.
Introduction
In recent decades, childhood obesity has significantly increased [1]. This rise in childhood obesity directly impacts how often and how severe sleep issues are in this group. Sleep-disordered breathing (SDB), which includes obstructive sleep apnea (OSA) and obesity hypoventilation syndrome (OHS), is the most common sleep problem in children with obesity. Previous studies found that OSA prevalence in children with obesity is much higher at 13%–71% [27] compared to the general pediatric population, estimated at 1%–6% [8]. OHS prevalence in adults ranges from 2%–29% [9,10], but data on children is limited.
Limited research has found other sleep problems, like circadian rhythm sleep-wake disorder, parasomnia, and polysomnographic features, specifically in children with obesity. These sleep problems also affect the duration and quality of their sleep. Each of these can impact health and social well-being over the long term. Research indicates a connection between not getting enough sleep and a higher risk of obesity over time [11-15]. Sleep problems that shorten sleep time can significantly contribute to the development of obesity. Disturbed sleep patterns are independently linked to the risk of metabolic and cardiovascular illness. It is also connected to changes in eating habits and visceral adiposity. Getting more sleep may also improve children's chances of success in a weight loss program [16].
It is crucial to understand the prevalence of sleep problems, particularly the polysomnographic features of children with obesity, to accurately quantify health risks, especially in cases of severe OSA. Understanding the spectrum of sleep disorders in children with obesity will support earlier detection of sleep problems and facilitate new approaches to improve sleep and reduce adverse consequences. To support this understanding, this study aimed to explore the demographic and polysomnographic features of children with obesity and determine if BMI can predict severe OSA in obese children.
Methods
1. Participants and study design
This cross-sectional study included children with obesity 3–18 years of age with symptoms suggestive of sleep disorders who underwent diagnostic polysomnography (PSG) at the Siriraj Sleep Center in Bangkok, Thailand, between January 2019 and March 2022.
For children under 5 years of age, obesity is defined as a weight for height more than 3 standard deviations above the World Health Organization (WHO) growth standard. For children over 5, obesity is defined as a body mass index (BMI) more than 2 standard deviations above the WHO growth standard [17,18], and morbid obesity is defined as a BMI greater than 3 standard deviations [19]. We excluded children with complex comorbidities including neuromuscular diseases, craniofacial disorders, and genetic metabolic and storage diseases.
Regarding comorbidities, adenotonsillar hypertrophy was diagnosed by an increase in adenoid or tonsillar tissue size as demonstrated by lateral skull film. Adenoid nasopharyngeal ratio was measured according to the method of Fujioka et al. [20] as shown in Fig. 1 [21]. Allergic rhinitis was diagnosed from chronic nasal symptoms (sneezing, nasal itch, watery rhinorrhea, and nasal blockade) together with identified causative allergens based on a positive skin prick test or serum allergen-specific IgE antibody measurements [22].

Adenoid-nasopharyngeal ratio measured by the Fujioka method. B line, straight line of anterior margin of basioccipital; adenoid (A), maximal diameter of adenoid measured perpendicular to B line; nasopharynx (N), distance between posterosuperior edge of hard palate and anteroinferior edge of sphenobasioccipital synchondrosis.
2. Measurements
We gathered information about demographics, medical history, and anthropometric parameters, and conducted a physical exam before children had PSG. We noticed 4 symptom categories. The first involved breathing issues during sleep, like snoring, mouth breathing, witnessed apnea, restless sleep (including leg kicks), nighttime awakening/sleep disturbance, morning headache, enuresis, and sweating during sleep. The second category included issues with staying awake during the day, like excessive daytime sleepiness (EDS), poor school performance, and concerns with concentration, mood, or behavior. The third category was related to circadian rhythms, including trouble falling asleep. The fourth category covered parasomnia, including nightmares, sleepwalking, and sleep terrors.
To assess the subjects’ anthropometry, body weight was measured using a digital scale, and height was measured using a stadiometer. Subsequently, BMI was calculated. A visual grading of tonsillar size was performed (0–4+, based on the Brodsky [23] criteria), with significant tonsillar hypertrophy defined as a rating of ≥2+.
Diagnostic PSG included the determination of sleep state through electroencephalography, electrooculography, and submental electromyography. The respiratory event was assessed through nasal/oral airflow using a thermistor, nasal pressure, respiratory inductance plethysmography monitoring chest and abdominal wall movements, pulse oximetry and transcutaneous CO2 (TcCO2) monitoring. Cardiac monitoring was also performed with electrocardiography.
Analysis of PSG data was completed by certified sleep specialists. Apnea-hypopnea index (AHI) was calculated according to the number of apneas and hypopneas during sleep divided by the total sleep time (TST). The oxygen desaturation index (ODI) was calculated according to the number of desaturation events ≥3% during sleep divided by the TST. The obstructive AHI index ≥1 events/hr was used to define the presence of OSA. The severity of OSA was characterized as mild (AHI, 1–4.9 events/hr), moderate (AHI, 5.0–9.9 events/hr), and severe (AHI, ≥10 events/hr) [24]. OHS was defined as a combination of obesity (body mass index≥the 95th percentile for age), daytime hypercapnia (arterial carbon dioxide tension≥45 mmHg), and SDB, after ruling out other disorders that may cause alveolar hypoventilation. Positional OSA was defined as a ratio of AHI in the supine position to AHI in a nonsupine position of ≥2 [25]. Rapid eye movement (REM)-related OSA was defined as a ratio of AHI in REM sleep to AHI in NREM sleep of >2 [24]. Sleep-related hypoventilation was identified by a CO2>50 mmHg for >25% of the TST. Periodic limb movements of sleep were defined as periodic limb movements in sleep index >5 events/hr [26].
3. Statistical analysis
Statistical analysis was performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA). The normality of numerical data was assessed using the Shapiro-Wilk test. Normally distributed continuous variables were summarized using mean±standard deviation, and non-normally distributed continuous variables were summarized as median (interquartile range [IQR]). Categorical variables were shown as numbers and percentages. We compared the variables between groups with AHI<10 events/hr and ≥10 events/hr using the Student t test or the Mann Whitney U test for numerical variables. Categorical variables were analyzed using the chi-square test or Fisher exact test. We used Spearman correlation to assess the association between anthropometric variables and PSG indices. We conducted logistic regression analyses to identify predictors associated with severe OSA in obese children. We used receiver operating characteristic (ROC) curves and binary logistic regression analysis to determine the optimal cutoff values of anthropometric parameters including BMI, and neck and waist circumference for prediction of severe OSA—a 2-tailed P value of <0.05 indicated statistically significant results. The study was approved by the Siriraj Institutional Review Board (approval no. Si 456/2021).
Results
1. Demographic
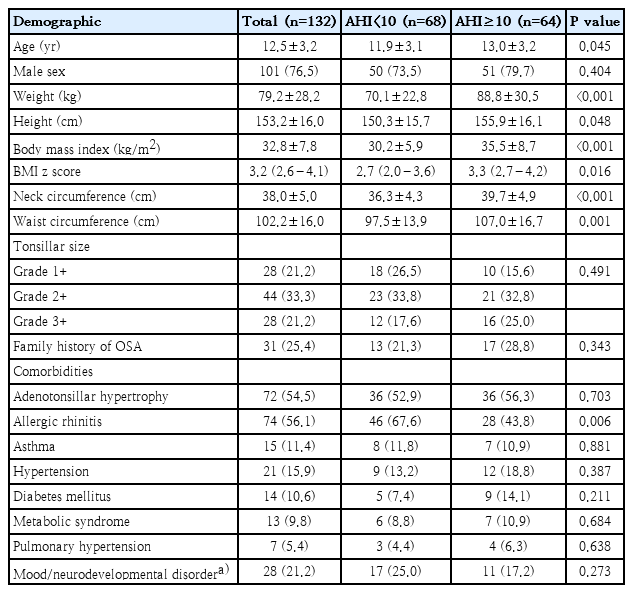
In this study, a total of 132 children with obesity were included. Their mean age was 12.5±3.2 years, and of those, 76.5% were male. The mean BMI was 32.8±7.8 kg/m2, with 39% classified as obese and 61% as morbidly obese. Additionally, 25.4% had a family history of OSA. At least one comorbidity was reported in 78.0% of the children. The common comorbidities included allergic rhinitis (56.1 %), adenotonsillar hypertrophy (54.5%), mood/neurodevelopmental disorders (21.2%), hypertension (15.9%), and asthma (11.4%). Among those with allergic rhinitis, they were sensitized to dust mite (75.7%), German cockroach (20.3%), American cockroach (13.5%), Bermuda grass (13.5 %), cat (10.8%), dog (8.1%), Alternaria (5.4%), Acacia (5.4%), Johnson grass (5.4%), Aspergillus (2.7%), and Curvularia (1.4%). In the study population, 34% of children reported regularly taking medications. Of those, they took inhaled corticosteroids (65.8%), antihypertensive drugs (15.9%), antiglycemic drugs (13.0%), bronchodilators (11.4%), and thyroxin (6.1%).
Age, weight, height, BMI, neck circumference, and waist circumference in the group with AHI≥10 events/hr were significantly higher than the group with AHI<10 events/hr (P<0.05) (Table 1).
2. Sleep duration and presenting symptoms
The median (IQR) of sleep duration was 9.0 (8.0–9.0) hours in children aged 35 years, 9.0 (8.0–9.5) hours in children aged 6–12 years, and 7.0 (6.5–8.0) hours in children aged 13–18 years. Additionally, 55% of the children had insufficient sleep for their age. The majority (76.0%) presented with multiple sleep problems. The most commonly reported sleep problems in this population were habitual snoring (91.7%), nocturnal mouth breathing (42.4%), EDS (36.4%), witnessed apnea (31.8%), and restless sleep (including leg kicks) (28.0%). There were more children with witnessed apnea in the group with AHI≥10 events/hr (P<0.05) (Table 2).
3. Polysomnographic results
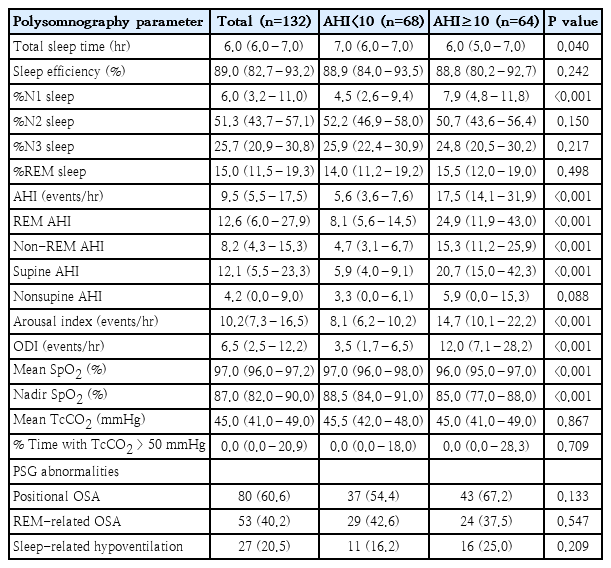
OSA was identified in 98.5% of children, of whom 18.2% had mild, 31.8% had moderate, and 48.5% had severe OSA. Overall, 51.5% of children had AHI<10 events/hr while 48.5% had AHI≥10 events/hr.
Not only did the children with AHI≥10 events/hr had significantly higher overall AHI, but they also had higher REM AHI, NREM AHI, and supine AHI (P<0.001). Additionally, children with AHI≥10 events/hr also exhibited lower TST (P=0.041), higher %N1 sleep (P<0.001), a higher arousal index (P<0.001), a higher ODI (P<0.001), and lower mean and minimum SpO2 (P<0.001).
Desaturation was observed in 59.8% of the children, 23.5% had hyperarousal, and 20.5% had sleep-related hypoventilation. Concerning OSA characteristics, 60.6% had positional OSA, and 40.2% had REM-related OSA. Additionally, OHS was diagnosed in 5.0% of the children (Table 3).
4. Anthropometric variables and ODI as predictors of severe OSA in obese children
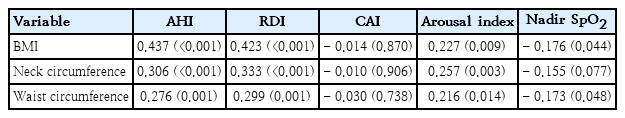
The BMI was positively correlated with AHI (r=0.437, P<0.001) and RDI (r=0.423, P<0.001) (Table 4). When determining whether anthropometric variables can predict severe OSA, BMI emerged as a significant predictor (odds ratio [OR], 1.11; 95% CI, 1.05–1.17; P<0.001). Additionally, neck circumference (OR, 1.15; 95% CI, 1.07–1.25; P<0.001), and waist circumference (OR, 1.04; 95% CI, 1.02–1.07; P=0.001) were also identified as significant predictors of severe OSA (Table 5).
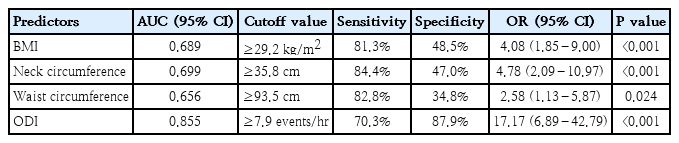
The ROC analysis of BMI for predicting severe OSA had the area under the curve of 0.689 (95% CI, 0.599–0.780; P<0.001) (Fig. 2A). The proposed BMI in predicting severe OSA is more than 29.2 kg/m2, with 81.3% sensitivity and 48.5% specificity (Table 6).

Receiver operating characteristic curves for predicting severe OSA. (A) Body mass index. (B) Neck circumference. (C) Waist circumference. (D) Oxygen desaturation index. AUC, area under the curve; CI, confidence interval; OSA, obstructive sleep apnea; ROC, receiver operating characteristic.
Prediction of severe obstructive sleep apnea in obese children using anthropometric variables and oxygen desaturation index
The ROC analysis of neck circumference for predicting severe OSA had the area under the curve of 0.699 (95% CI, 0.609–0.788; P<0.001) (Fig. 2B). The proposed neck circumference in predicting severe OSA is more than 35.8 cm, with 84.4% sensitivity and 47.0% specificity (Table 6).
The ROC analysis of waist circumference for predicting severe OSA had the area under the curve of 0.656 (95% CI, 0.563–0.749; P=0.002) (Fig. 2C). The proposed waist circumference in predicting severe OSA is more than 93.5 cm, with 82.8% sensitivity and 34.8% specificity (Table 6).
The ROC analysis of ODI for predicting severe OSA had the area under the curve of 0.855 (95% CI, 0.791–0.920; P<0.001) (Fig. 2D). The proposed ODI in predicting severe OSA is more than 7.9 events/hr, with 70.3% sensitivity and 87.9% specificity (Table 6).
Discussion
Children with obesity undergoing PSG at our sleep center, encompassing both prepubertal and pubertal groups, predominantly male, and with various comorbidities, were primarily referred by general pediatricians for a comprehensive OSA evaluation. A subset of this cohort exhibited signs of severe OSA, underscoring the widespread impact of this sleep disorder among pediatric patients with obesity.
Previous research by Andersen et al. [8] in 2016 investigated the prevalence of OSA in children with obesity, reporting rates ranging from 19% to 61%. The prevalence of OSA in those undergoing PSG assessments varies between 13% and 71% [2-7]. Our study focused on children with obesity undergoing PSG in our center and revealed that 98.5% of these subjects displayed OSA symptoms, with 48.5% diagnosed with severe OSA. The difference may be due to variations in the study population and the threshold for referral and consideration of diagnostic PSG in obese children in each country.
Our study noted a significant prevalence of positional OSA in children with obesity, suggesting the potential effectiveness of positional therapy as a management strategy. The increased prevalence and severity of OSA observed are partly attributed to the escalating childhood obesity severity in Thailand [1,27]. This temporal correlation underscores a substantial rise in severe OSA incidence, reflecting the broader health implications of the obesity epidemic in pediatric populations.
Moreover, our study highlights a systemic issue contributing to delayed diagnoses and worsening consequences of pediatric OSA - an overall lack of awareness coupled with obstacles in the referral system. Although PSG is the gold standard for the diagnosis of OSA, the limited resources of pediatric sleep centers and pediatric sleep specialists pose challenges in diagnosing OSA in Thai obese children. These factors collectively impede timely interventions and emphasize the necessity for heightened awareness and streamlined referral processes in the healthcare system.
Identification of OSA requires attention to both nighttime and daytime symptoms. In our study, common nighttime symptoms included habitual snoring, nocturnal mouth breathing, and witnessed apnea. Similarly, prevalent daytime symptoms comprised EDS, below average school performance, and concerns about reduced concentration, mood disturbances, or altered behavior. Notably, our study found a higher prevalence of habitual snoring but a lower frequency of EDS compared to a study on Canadian children with obesity by MacLean et al. [5]. Witnessed apnea was the most suggestive symptom of severe OSA. Interestingly, within our severe OSA cases, some children exhibited the condition without the typical symptom of habitual snoring leading to the challenge in diagnosis.
The children with severe OSA were significantly older than the other group. The study of Kohler et al. [28] revealed an increased susceptibility to OSA among adolescents, particularly in association with obesity. OSA's etiology involves a complex interplay of physiological and anatomical factors, varying across populations. Our findings indicated a male predominance among children with obesity with OSA, aligning with the preceding adult-focused study [29]. National Canadian data from 2004 to 2013 consistently showed a higher obesity prevalence in boys aged 3 to 19 [30]. Previous studies emphasized the influence of sex hormones on leptin production and release at the adipocyte level. Males with higher androgen levels experience a suppressive effect, resulting in lower concentrations of leptin in their bloodstream compared to females [31]. Lower serum leptin may increase susceptibility to obesity. However, an investigation by Horne et al. [32] found no gender disparities in SDB severity or consequences in children, like our findings.
In our study, children with obesity form a specific subgroup with complex comorbidities. Adenotonsillar hypertrophy and obesity are key risk factors for OSA in children. Adenoid and/or tonsil hypertrophy is currently recognized as the primary risk factor for OSA in children, narrowing the upper airway and potentially causing severe dynamic airway blockage during sleep, especially when combined with factors like decreased muscle tone. Childhood obesity-related OSA differs from OSA in otherwise healthy children with adenotonsillar hypertrophy.
Based on our findings, we proposed a distinction between type 1 OSA (marked by adenotonsillar hypertrophy without obesity) and type 2 OSA (linked to obesity) [33]. Adenotonsillar hypertrophy was the primary comorbidity in our study of children with obesity; however, it was not significantly correlated with OSA severity. In contrast to the study of Katharina et al., which found an association between increasing BMI and OSA severity exclusively in children aged 7 years and older [34], our research identified a substantial link between elevated BMI and OSA severity across all age groups.
Other risk factors for pediatric OSA, such as allergic rhinitis, asthma, preterm birth, male sex, parental smoking, apnea symptoms, and ethnicity, exhibit diverse reporting [35]. In our study, allergic rhinitis emerged as a prevalent comorbidity, linked to OSA through inflammation and partial nasal airway blockage due to increased mucus secretion and edema.
Apart from OSA, adults with obesity also face heightened levels of EDS. They show decreased sleep efficiency and a lower percentage of REM sleep on PSG in comparison to individuals without obesity. The assertion underscores that OSA alone falls short of fully elucidating the source of EDS.
Furthermore, children with obesity also experience various sleep disorders, including circadian rhythm sleep-wake disorders, reported in 25.8% of children with obesity in French [36]. This prevalence, notably higher than in our study, disrupts sleep efficiency and shortens sleep duration, increasing the risk of obesity [37]. In our investigation, 55% of participants exhibited insufficient sleep for their corresponding age groups. Chaput [38] 2016 study underscored the association between sleep deprivation and diverse health conditions, including obesity, cardiovascular disease, and diabetes. Our study indicated a tendency for reduced sleep duration in children with obesity with severe OSA. Previous research suggested that children with shorter sleep durations had a significantly higher OR for overweight/obesity [39,40], establishing a positive correlation with sleep deprivation, blood glucose fluctuations, negative mood, and cravings [41]. These factors collectively contribute to increased caloric intake, ultimately leading to obesity.
The study explored various parasomnias, including nightmares (5.3%), sleepwalking (3.0%), and sleep terrors (2.3%). Nightmares and night terrors differ in occurrence during distinct sleep stages. Nightmares are more frequent during REM sleep, associated with vivid dreaming. Children wake up from nightmares with recollection. In contrast, night terrors happen during deep non-REM sleep. Children experiencing night terrors may talk and move but cannot recall the dream. Night terrors are common in children aged 3 to 8, while nightmares can affect children of all ages. Notably, our study found no association between these parasomnias and the severity of OSA.
Uncertain processes contribute to sleep issues in individuals with obesity, distinct from OSA. Inadequate sleep duration is a notable risk factor for obesity. However, a comprehensive investigation is essential to understand the mechanisms linking obesity with sleep disorders beyond OSA. Additionally, further research is required to assess the impact of therapeutic interventions on improving sleep quality in children with obesity.
Our investigation found a significant decrease in TST and an increase in N1 sleep among pediatric subjects with severe OSA. This group exhibited a notably elevated AHI during both REM and non-REM sleep, especially in the supine position, along with an increased arousal index and more severe desaturation. N1 sleep, known as 'active sleep,' constitutes around 4%–5% of TST in typical pediatric subjects [42]. A 2016 study in adults with OSA observed increased N1 sleep, normal N2 sleep, reduced N3 and REM sleep, and frequent arousals [43]. After adjusting for confounding variables like age and BMI, the prevalence of N1 sleep remained significantly higher in the OSA patients compared to both primary snoring and normal control groups [44].
OSA in children worsens during REM sleep, with a concurrent rise in the respiratory arousal index in the latter third of the night, associated with increased REM sleep [45,46]. In our study, we found a lower prevalence of REM-related OSA and a higher prevalence of positional OSA compared to our prior research involving a broader pediatric population [47].
Limited access to PSG in Thailand, marked by extended waiting lists and financial barriers, contributes to delayed diagnosis and treatment of OSA among children with obesity, increasing severity and risk of adverse outcomes. According to our findings, we recommend screening children with obesity with a BMI greater than 29.2 kg/m2, a neck circumference greater than 35.8 cm, or a waist circumference greater than 93.5 cm for severe OSA using sleep questionnaires and PSG.
In regions without easy PSG access, overnight oximetry is a potentially valuable diagnostic tool for identifying children with obesity with severe OSA. The widely used McGill Oximetry score aids early OSA detection in children suitable for adenotonsillectomy. Importantly, only 2% of the validation sample comprised children with obesity [48]. Moreover, the McGill criteria demonstrated reduced sensitivity and specificity for diagnosing OSA in the context of obesity [49]. The ODI reliably predicts severe OSA in children with obesity. In Canadian children with obesity, an ODI≥6 events/hr predicted moderate/severe OSAS with an OR of 6.29 (95% CI, 2.82–14.06; P<0.001), and sensitivity and specificity both at 71% [5]. Recognizing ODI cutoff variability across populations is crucial. In our study, we proposed ODI more than 7.9 events/hr in predicting severe OSA, with 70.3% sensitivity and 87.9% specificity. Additionally, ODI from overnight oximetry may differ from PSG, emphasizing the need for meticulous PSG scrutiny with precise probe placement and supplementary signals for artifactual oximetry removal.
Our study had some limitations. Our data from children with obesity undergoing PSG may not be representative of the children with obesity in the general pediatric population.
In conclusion, severe OSA is common in children with obesity. We recommend screening children with obesity with a BMI greater than 29.2 kg/m2 using sleep questionnaires and PSG.
Notes
Conflicts of interest
No potential conflict of interest rele vant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or notfor profit sectors.
Author contribution
Conceptualization: RS, PTovichien; Data curation: RS, PTiamduangtawan; Formal analysis: RS, PTovichien; Methodology: RS, PTovichien, KU, WC; Writing original draft: RS, PTovichien; Writing review & editing: PTovichien, KU, WC
Acknowledgements
The authors would like to thank the studied participants and their parents for generously agreeing to participate in this study. We are also grateful to sleep technicians, nurses, and healthcare workers at Siriraj Sleep Center who provided insight and expertise that greatly assisted the research. We gratefully acknow ledge Siriraj Institute of Clinical Research (SICRES) for suppor ting the manuscript development, Mark Simmer man for editing the manuscript, and Kanokwan Sommai for statistical analysis.