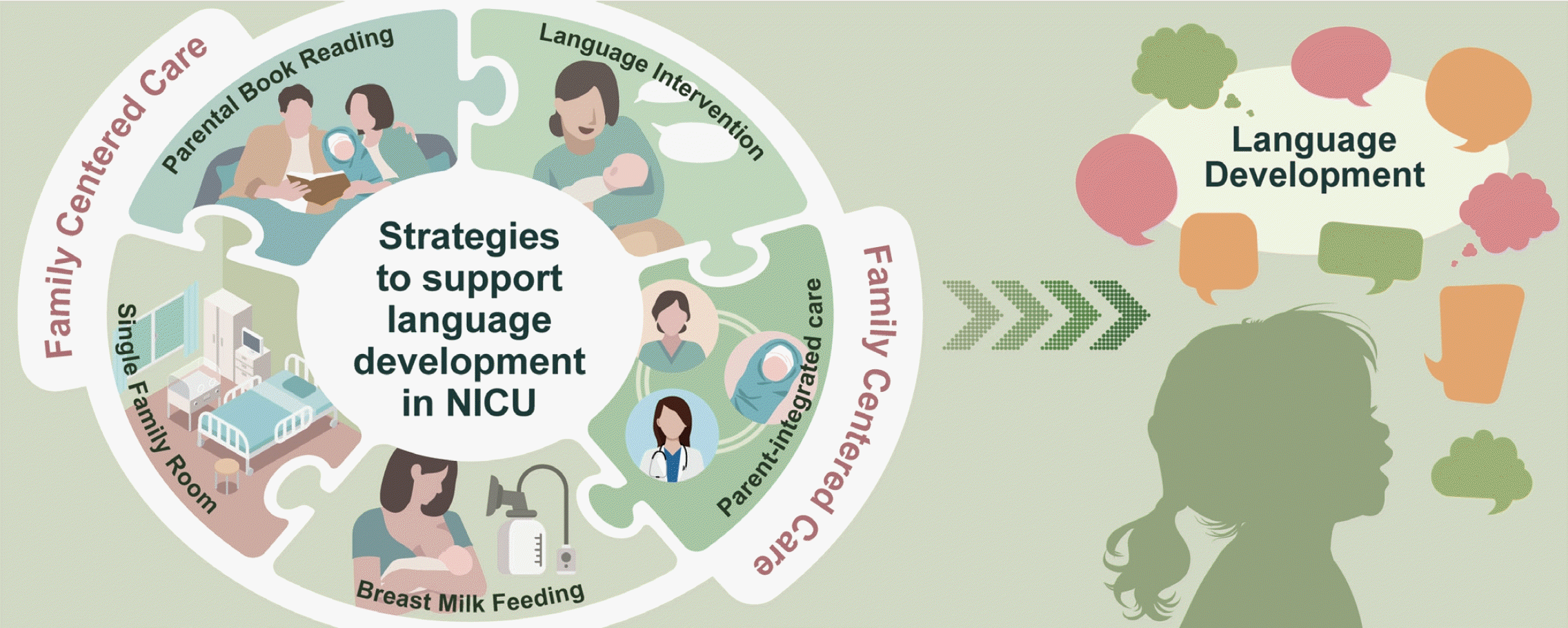
Strategies to support language development in neonatal intensive care unit: a narrative review
Article information
Abstract
Despite neonatal intensive care advancements and quality improvements, preterm infants often experience delays in speech and language development during early childhood. The etiological pathway of language delays is multifactorial, including younger gestational age at birth, male sex, pregnancy complications including gestational diabetes mellitus and preeclampsia, organic pathology from neonatal morbidities, environmental factors of the neonatal intensive care unit (NICU) and prolonged hospitalization, home environment including socioeconomic status and parental education, and parent-infant interactions. As early language experiences and environments are crucial for the development of language processing, strategies to support language development should be implemented from the NICU onward. This study aimed to summarize evidence- based strategies for language development through an extensive review of nutrition, NICU environment, language and sound exposure, developmental care interventions, and family-centered care. Promoting breastfeeding, increasing parent-infant interactions in a single-family room setting, nurturing the language environment via parental book reading and language interventions, and parent-integrated interventions in the NICU could potentially enhance language development among preterm infants. These supportive strategies can be integrated through family-centered care, which recognizes parents as primary caregivers and collaborative partners.
Key message
· Preterm infants often experience speech and language development delays during early childhood, impacting children's ultimate outcomes.
· Promoting breastfeeding, increasing parent-infant interactions in a single-family room, promoting a nurturing language environment by parental book reading and language interventions, and parent-integrated interventions in the neonatal intensive care unit could potentially enhance children's language development.
· Integrating these strategies through family-centered care is essential.
Graphical abstract. Strategies for supporting language development in neonatal intensive care unit (NICU).
Introduction
Early language development is crucial because it serves as the foundation for basic communication, cognitive process, literacy, and social interactions [1]. Language delays have profound and lasting impacts on children's ultimate outcomes. Preterm infants often face speech and language delays during early childhood that are often associated with other deficits including hearing loss, cognitive deficits, behavioral abnormalities, and cerebral palsy [2-4]. A prospective Canadian national cohort study demonstrated that language delays, defined as Bayley Scales of Infant and Toddler Development, 3rd edition (BSID-III) composite scores of <85, occurred in 35.3% of very preterm children [5]. According to a recent large-scale study of a national Korean cohort, the risk of developmental delay occurring by 6 years of age increased with younger gestational age at birth. In particular, preterm infants born before 28 weeks' gestation had a 3.57× higher risk of language disorders than full-term infants [6]. Since language relies on the brain's complex structure and function, alterations in which are significant indicators of neurodevelopment impairment [7]. Although survival and severe neurodevelopmental impairment have improved due to neonatal intensive care advancements and quality improvements, minimal advancements in language difficulties have been noted [8].
The etiological pathway of language delays is most likely multifactorial, including younger gestational age at birth and male sex [5,6,9]; pregnancy complications including gestational diabetes mellitus and preeclampsia [7]; organic pathologies from neonatal morbidities including bronchopulmonary dysplasia, brain injury, necrotizing enterocolitis and severe retinopathy of prematurity [5,9-12]; neonatal intensive care unit (NICU) environmental factors and prolonged hospitalization [13-15]; home environment including socioeconomic status and parental education [1,9,16-18]; multicultural families [19,20]; and parent-infant interactions [21-26]. Because early language experience is necessary for normal speech and language processing development, limited early language exposure in the NICU can be a significant cause of language delays among preterm infants [13,27,28].
Neuroplasticity, also known as brain plasticity, refers to the ability of the nervous system to change its activity in response to intrinsic or extrinsic stimuli by reorganizing its structure, functions, or connections [29]. Neuroplasticity is among the primary mechanisms through which humans adapt to environmental changes and demonstrate resilience to adverse events [4]. There may be a “sensitive period” in which neural circuits are particularly sensitive to environmental pressures [30]. In particular, the preterm and newborn periods, during which the development of brain connections and synaptogenesis occurs vigorously, involve greater plasticity [31]. The high plasticity of the brain during these early stages implies that positive experiences provided in an infant's environment can provide opportunities to positively influence cognitive and language outcomes [32]. For this reason, providing a nurturing language environment and appropriate access to aural or visual language in the NICU are critical for language development in preterm infants [21].
Broadly, this review centers on strategies that support language development in the NICU. Moreover, it provides an abridged overview of nutrition, language and sound exposure, parent-integrated interventions, and family-centered care in the NICU environment.
Nutrition
Early nutrition is critical to an individual's growth and development, and exclusive breast milk (BM) feeding is universally recommended for the health and well-being of all infants [33]. Preterm infants are at high risk for caloric and nutrient deficiencies, which can negatively affect their development. The critical nutrients found in BM, such as polyunsaturated fatty acids (PUFAs) and other neurotrophic factors, could have positive impacts on the brain development of preterm infants. Therefore, BM for preterm infants is emphasized even more.
The impact of BM, donor BM, and supplemental docosahexaenoic acid (DHA) provided to either mothers or neonates on infants' language development will be reviewed below.
1. Breast milk
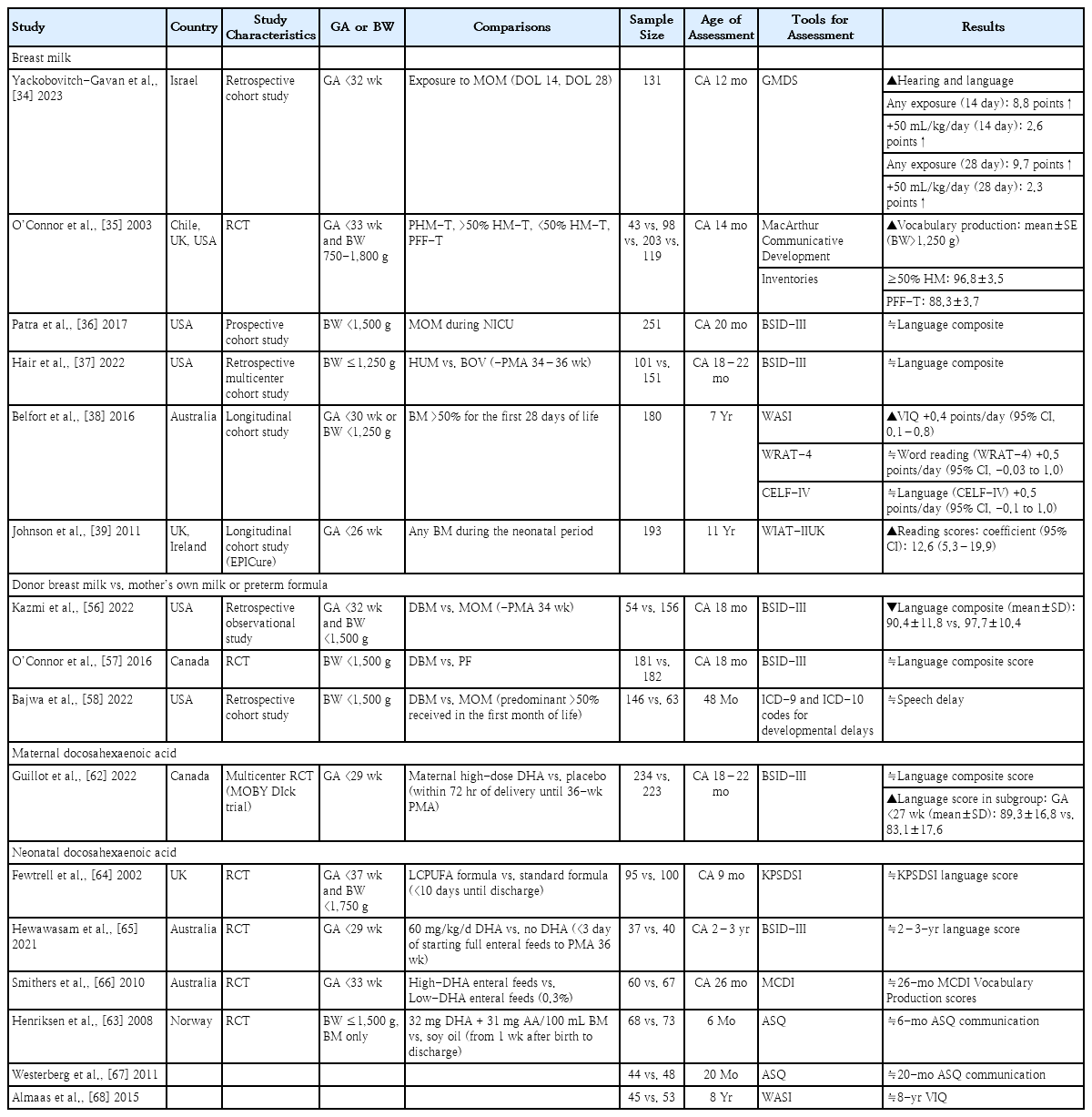
Several studies have assessed language outcomes following BM exposure in the NICU. Table 1 presents the impact of BM based on assessment age. Studies investigating the impact of BM on the language development at 12–14 months of corrected age (CA) reported positive effects [34,35]. In particular, Yackobovitch-Gavan et al. [34] reported that early exposure to the mother's own milk (MOM) at postnatal days 14 and 28 enhances hearing-language scores at a CA of 12 months in very preterm infants. Studies evaluating language developmental outcomes at a CA of 18–22 months using the BSID-III found no significant differences in language composite scores between the BM and non-BM groups [36,37]. Belfort et al. [38] evaluated the associations of BM intake by very preterm infants with neurological outcomes at 7 years of age. They concluded that predominant BM feeding in the first 28 days of life was associated with a better verbal intelligence quotient. However, no significant correlation was noted between breastfeeding and reading or language scores. In Johnson's study of the early predictors of educational outcomes of extremely preterm infants at 11 years of age, the receipt of any BM during the neonatal period was an independent predictor of high reading scores [39].
Limitations were noted in assessing the impact of breastfeeding during the early postnatal period due to inter-study variations among study subjects; breastfeeding definitions, timing, and duration; neurodevelopmental evaluation timing; and assessment tools. Nevertheless, the effect of BM seems stable from early life throughout childhood.
While the mechanisms explaining the relationship between BM and language development have not been extensively researched, several possibilities should be considered. Postnatal brain development may be sensitive to dietary elements because of the relatively permeable nature of the infant gut and blood-brain barrier to small molecules [40]. Previous studies demonstrated that BM affects neural tissue structure and gray- and white-matter maturation and myelination, leading to favorable neurocognitive outcomes [38,41-44]. The fatty acid profiles of BM might play a significant role in brain structure and function. PUFAs, especially omega-3 (DHA) and omega-6 (arachidonic acid), are involved in neurogenesis, neuronal migration, gliogenesis, synaptogenesis, and myelination from fertilization [45-48]. This process may shape the neural pathways linked to the developmental progression of language acquisition [49].
Other constituents of BM, including micronutrients and bioactive components such as human milk oligosaccharides, hormones, growth factors, and immunoglobulins, may contribute to brain development and work synergistically to optimize neurodevelopmental outcomes [50-53]. Moreover, BM may impact neurodevelopment via epigenetic mechanisms. Its bioactive compounds, such as noncoding RNAs, stem cells, and microbiomes, are under study as epigenetic modulators that influence neurodevelopment [54]. Furthermore, breastfeeding, whether directly or via exclusive pumping, stimulates the secretion of maternal pituitary hormones, particularly oxytocin. These oxytocin changes persist for an extended period and may affect maternal behavior and the motherhood experience as well as influence infant cognition, learning, and language developmental outcomes [51,55].
2. Donor BM
With an increasing awareness of the positive effects of BM on neonatal growth and development, the use of pasteurized donor BM has become increasingly popular in the NICU when the MOM is unavailable. Several studies have assessed the long-term effects of donor BM on language development (Table 1). Kazmi et al. [56] reported that infants fed donor BM had lower language scores at CA of 18 months compared to those fed MOM. In a randomized controlled trial (RCT) comparing donor BM and preterm formula for 90 days or until discharge, the use of supplemental donor BM did not improve language development among very low birth weight (VLBW) infants at a CA of 18 months [57]. In a retrospective cohort study evaluating neurodevelopment at 48 months of age, no significant differences were found in the risk of speech delay between infants who received donor BM versus MOM [58]. Thus, donor BM did not have a positive impact on long-term language development.
Donor BM may not offer similar developmental benefits to MOM due to several factors, including the compositional differences between the milk of mothers of preterm versus full-term infants [59,60], the loss of certain nutrients during the pasteurization, storage, and processing of donor BM [59,61], and the lack of hormonal changes in the mother with milk expression.
3. Docosahexaenoic acid
Lipids, accounting for more than 50% of the cerebral tissue’s dry weight, are the nutrient deemed most closely related to brain development. DHA (C22:6n-3) is an essential long-chain PUFA in nervous system formation and function, particularly the brain and retina. DHA is currently regarded a crucial nutrient during pregnancy and breastfeeding due to its active involvement in early nervous system development [47].
Maternal DHA supplementation
A multicenter RCT evaluated the effect of maternal high-dose DHA on the language neurodevelopment of very preterm infants at a CA of 18–22 months (Table 1) [62]. Maternal DHA supplementation did not improve the language outcomes of breastfed preterm infants. However, a subgroup analysis suggested a potential benefit of maternal DHA for the language development of preterm infants born before 27 weeks’ gestation. Additional well-designed research is needed to confirm this finding.
Neonatal DHA supplementation
Table 1 shows several RCTs that evaluated the effects of neonatal DHA supplementation on language developmental outcomes. Two demonstrated neurodevelopmental effects within 1 year of age [63,64]. Henriksen et al. [63] reported that DHA supplementation for BM-fed preterm infants did not improve the communication subtest scores of the Ages and Stages Questionnaire. Fewtrell et al. [64] also showed no significant difference in Knobloch, Passamanick and Sherrard’s Developmental Screening Inventory language scores between long-chain PUFA and standard formula-fed groups. In studies assessing language development at a CA of 18 months to 3 years, no significant differences in developmental scores were noted between the groups supplemented withDHAand the control group [65-67]. Almaas et al. [68] administered the Wechsler Abbreviated Scale of Intelligence to 8-year-olds to evaluate the long-term effects of DHA supplementation during NICU hospitalization. Their findings revealed no significant difference in verbal intelligence quotient between the DHA group and the control group. Summarizing the current research findings, it is challenging to consider neonatal DHA supplementation as having positive effects on the language development of preterm infants.
NICU environment
The structural components of the ears develop in the first 20 weeks of gestation [69]. The human cochlea and peripheral sensory end organs normally develop fully by 24 weeks' gestation [70]. The auditory system typically becomes functional around 25 weeks' gestation. The hearing threshold is approximately 40 decibels A (dBA) at 27–29 weeks' gestation. It gradually decreases to a nearly adult level (13.5 dBA) by 42 weeks' gestation, indicating ongoing postnatal maturation of the auditory pathways of the central nervous system [71]. The period from 25 weeks' gestation to 5–6 months of age is crucial to the development of the neurosensory part of the auditory system [69].
The language and sound environment to which fetuses and preterm infants are exposed differs. The intrauterine environment is characterized by constant primarily low-frequency sounds from the mother's cardiovascular and digestive systems transmitted through the amniotic fluid [72,73]. Extrauterine sounds are altered as they pass through abdominal tissue, leading to some sound attenuation that may be more pronounced at high- and mid-frequency energy bands [72,73]. Language and extrauterine sound exposure for fetuses exhibited a marked cyclical day/night pattern with reduced exposure during nighttime hours [74]. In contrast, preterm infants in the NICU are minimally exposed to biological sounds; in fact, they may receive nearly 5×s less language exposure than fetuses [74]. Instead, they are exposed to high sound levels and electronic and mechanical noises, all of which are transmitted through the air rather than through fluid [75,76]. Such exposures vary significantly less throughout the 24-hour cycle [74]. These abnormal auditory exposures for preterm infants may contribute to speech/language deficits later in life [13,77].
1. Single-family room versus open-bay NICU
The traditional NICU was an open-bay environment of multiple beds located in a single space. However, most human conversations in an open-bay NICU design are blocked by ambient noise, which deprives infants of meaningful language stimulation. As a result, recent recommendations for NICU design include the construction of private spaces, referred to as single-family rooms (SFRs), to reduce ambient noise and facilitate family involvement [78]. However, single-room NICU designs also feature increased social isolation, causing language deprivation and atypical language development [13]. Therefore, it is important to evaluate language outcomes comparatively by NICU design.
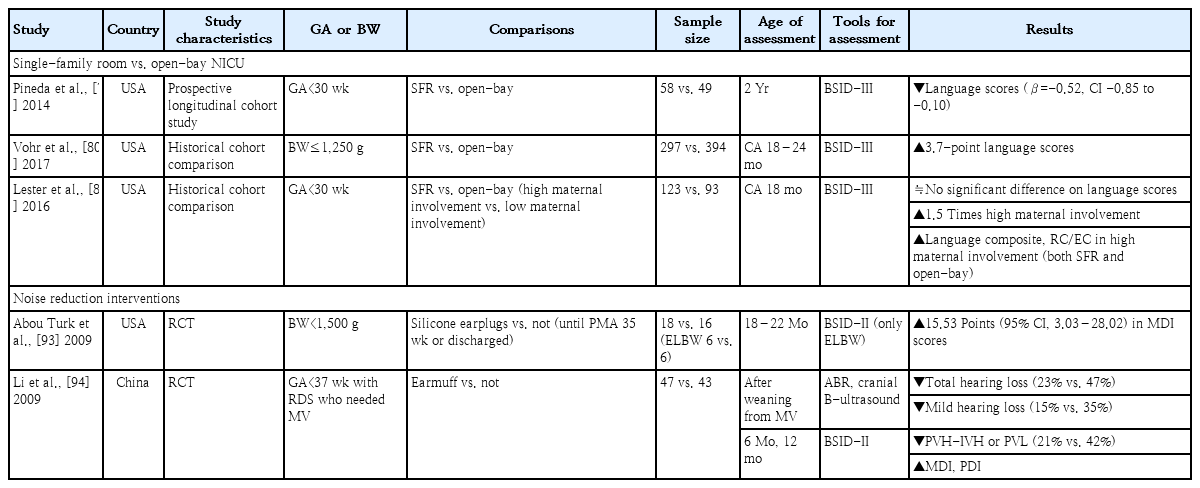
Regarding long-term language outcomes, several studies reported conflicting results (Table 2). In the prospective longitudinal cohort study of Pineda et al. [79], very preterm infants cared for in SFRs had reduced cerebral maturation at term-equivalent age and poorer language development at 2 years compared to those cared for in open bays. On the other hand, Vohr et al. [80] reported that the SFR NICU was associated with a 3.7-point increase in mean language score at a CA of 18–24 months. Lester et al. [81] reported that infants with high maternal involvement in the NICU (both SFR and open-bay designs) had greater language composite, receptive, and expressive communication scores than infants with low maternal involvement. However, the number of days of maternal involvement and proportion of high maternal involvement were greater in the SFR than open-bay NICU design. The current evidence is insufficient to support any specific NICU design due to lack of RCTs. Nevertheless, due to the significant positive impact of SFR on language development, driven by increased maternal involvement through family-integrated care, it is reasonable to actively consider SFR design for future NICU facilities [81,82].
2. Noise-reduction interventions
Overstimulation by the loud and noxious noises of the NICU has been well documented. According to American Academy of Pediatrics recommendations, safe sound levels in the NICU should not exceed an hourly level of 45 dBA [70]. However, the NICU noise level typically exceeds the recommended standards, often falling outside the acceptable range more than 70% of the time [75,83]. Long-term average equivalent sound levels (hourly, daily, or weekly) typically range from ~50 to 65 dBA in various NICU settings [75,84,85]. Short-term-equivalent sound level measurements can peak at 75–85 dBA [70,86]. This loud and noxious noise not only has negative short-term effects on the infant's cardiovascular and respiratory system, but it also exerts negative long-term effects on brain maturation and neurodevelopment [87-89]. Therefore, efforts to reduce noise in the NICU continue [90-92].
Only 2 studies have examined the long-term effects of noise reduction on the neurodevelopment of preterm infants (Table 2) [93,94]. One high-quality RCT that used silicone earplugs to reduce the sound level for VLBW infants showed the positive effects of earplugs on the Mental Developmental Index (MDI) at 18–22 months, especially only for extremely low birth weight infants [93]. However, the sample size was very small, with only 6 infants each in the earplug and control groups. In another RCT of preterm infants diagnosed with respiratory distress syndrome requiring mechanical ventilation, the group using earmuffs showed a significantly lower incidence of hearing loss and periventricular/intraventricular hemorrhage or periventricular leukomalacia than the control group. Additionally, the MDI scores at 6 and 12 months were significantly higher in the earmuffs group [94]. However, due to the small sample size, heterogeneous subjects, and variety of study methods used, it is difficult to draw a definitive conclusion regarding the noise-reduction effects of earplug or earmuff usage on language outcomes for preterm infants. Larger and well-designed trials are needed to verify results to date.
Language and sound exposure
During the last trimester of pregnancy, hearing becomes functional as previously discussed. In utero, low-frequency components of language, including pitch, as well as some aspects of prosody and rhythm are transmitted to fetuses through the uterus. Moreover, fetuses are exposed to properties of native language from their mother’s speech via bone conduction, aiding in early language acquisition [95]. This prenatal language experience shapes the neonatal neural response to speech [96]. Shortly after birth, neonates demonstrate specific responses to speech and can discern different prosodic patterns, indicating early tuning to the language environment [96,97]. Neuroimaging studies have provided considerable data about language development in neonates and young children. Perani et al. [98] demonstrated that, in 2-day-old infants, the language-related neural substrate was fully active in both hemispheres with a preponderance in the right auditory cortex. Neuroimaging conducted of 3-month-old infants indicated that speech processing relied on inferior frontal and temporal brain regions interconnected by 2 primary fiber bundles: the arcuate fasciculus and the uncinate fasciculus [99-101]. Minagawa-kawai et al. [102] delineated a clear left-lateralized cerebral basis for speech processing, predominantly to native language, in 4-month-old infants. Development of the speech production ability is associated with a notable increase in overall brain size. The most significant growth in cortical surface area occurs during the first and second years of life, with most notable growth of the superior temporal gyrus occurring during the first year of life [103,104]. In conclusion, a functional organization of the brain for language development is present from the fetal period to early infancy.
1. Parental book reading
Shared book reading is one of the most effective ways to enrich an infant's language and a well-recognized facilitator for higher language skills and literacy acquisition in preschoolers [105,106]. The American Academy of Pediatrics has issued a policy statement endorsing the commencement of parent-child home reading [107]. However, the impact of initiating parental book reading from the NICU setting on the subsequent language development of preterm infants with restricted language exposure remains unknown.
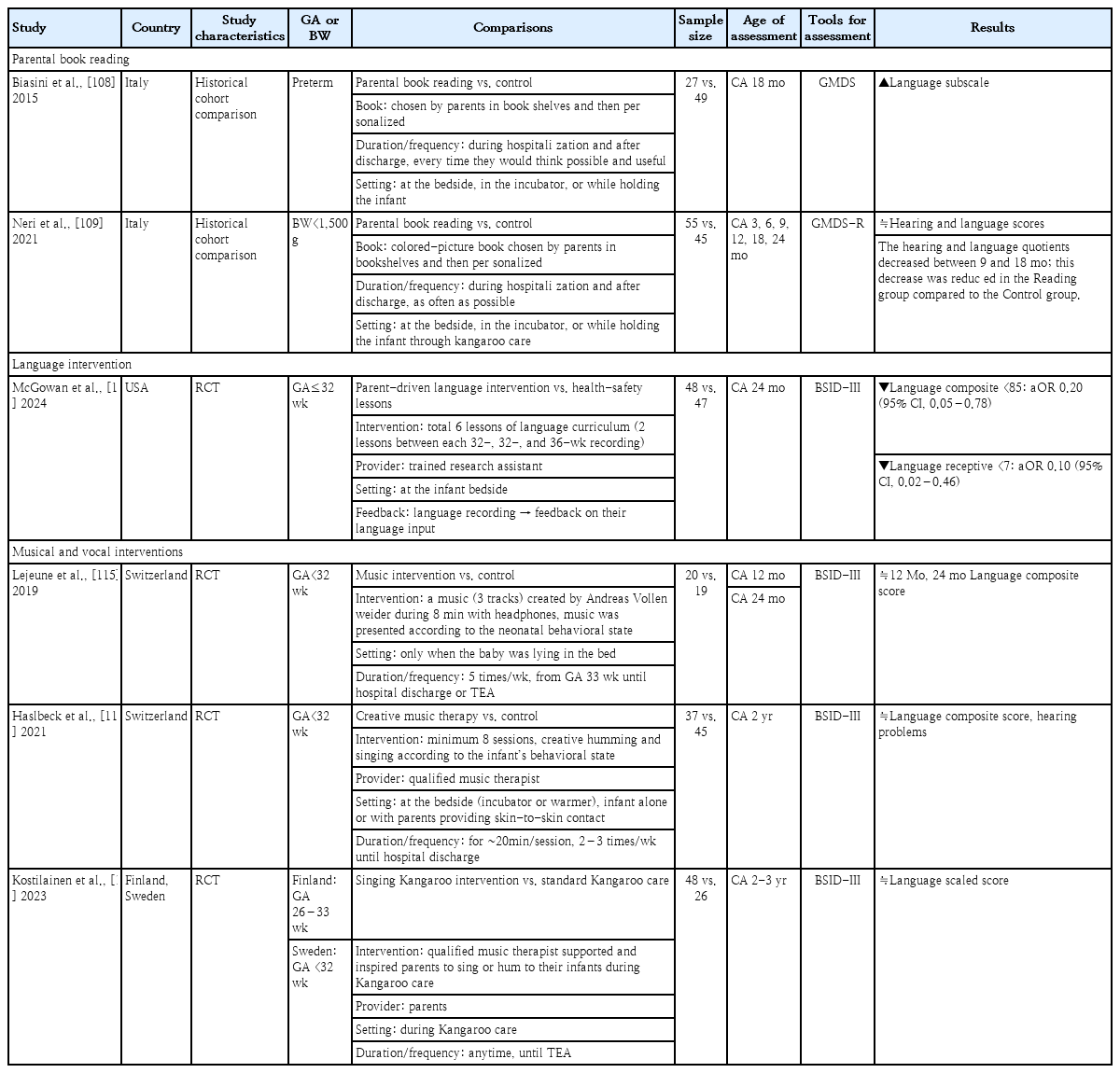
Two pre-post historical comparison studies from same institution evaluated the effects of parental book reading on the language development of preterm infants (Table 3) [108,109]. In both studies, the books were chosen by parents from bookshelves and then personalized. Parental book reading was conducted during the hospitalization, and the parents were encouraged to continue the practice after discharge. The frequency of book reading was not predetermined by a protocol; instead, the parents read books as often as possible. This was done at the bedside, in the incubator, or while holding the infant through kangaroo care. Biasini et al. [108] reported improved language scores for preterm infants at a CA of 18 months in the parental book reading versus control group. Neri et al. [109] examined VLBW infants and found no significant intergroup differences in hearing and language scores until a CA of 24 months. Both groups showed decreasing hearing and language quotients at a CA of 9–18 months. However, this decrease was reduced in the parental reading versus control group.
Parental book reading has positive effects on language development, not only by exposing infants to their parents' language but also by positively influencing the parent-infant interaction, reducing parental stress, and enhancing functional brain connectivity [110-113]. Moreover, regular parental book reading may be an epigenetic factor associated with later reading success [107].
2. Language intervention
In a recent RCT, the language development of a parent-driven language intervention was compared with those of health-safety lessons (control group) (Table 3) [114]. The intervention group received 6 lessons of known methods for early language intervention involving language activities for parents to complete with their infants that included topics such as reading, singing, and narration of bedside activities. Natural language data recordings were obtained using a Language Environment Analysis digital language processor at 32, 34, and 36 weeks of life. This device analyzes the number of words spoken by adults, the child's vocalizations, and conversational turns. Parents in the intervention group received feedback on their language input to their infant every 2 weeks based on each previous language recording. The intervention group had higher adult word counts at 36 weeks and demonstrated an increase in adult word counts between recordings. The intervention reduced the risk of having a language composite score of <85 and a receptive score of <7 on the BSID-III assessment at a CA of 24 months. Thus, a language intervention in the NICU that leads to an increased exposure to adult word counts, may positively impact the language development of preterm infants.
3. Musical and vocal interventions
Three RCTs assessed neurodevelopment by applying music therapy or singing (Table 3) [115-117]. The enrolled study participants were younger than 32–33 weeks' gestation. One study provided recorded music [115], while the other 2 studies offered songs/humming provided directly by music therapists or the parents [116,117]. The interventional duration and frequency varied among the studies. When evaluating language development at a CA of 12 months and 2–3 years, the studies found no significant intergroup differences in language scores. This comprehensive review of current research findings identified no clear evidence to substantiate the effectiveness of musical and vocal interventions for preterm infants.
Parent-integrated interventions
The NICU environment can induce stress for both parents and infants through early separation, potentially impeding early bonding and attachment [118]. Considering that parents are invaluable as the primary caregivers of and crucial advocates for their infants, there is a strong emphasis on early involvement of parents in the NICU to support their engagement in early caregiving and promote an adaptive post-discharge transition to home [119-121].
Two RCTs evaluated the neurodevelopmental outcomes of early parent-integrated interventions in the NICU (Table 4) [122,123]. The Family Nurture Intervention was designed to overcome the maladaptive conditioning effects of maternal separation and the NICU environment on preterm infants [122]. Nurture specialists facilitated the Family Nurture Intervention during mother-infant sessions. The first activity was a scent cloth exchange between the mother and the infant. Nurture specialists encouraged the mothers to engage in touching, communication, and maximizing eye contact with their infants during both skin-to-skin and non-skin-to-skin holding sessions. The Family Nurture Intervention significantly improved the cognitive and language scores of infants whose scores were greater than 85 at a CA of 18 months [122].
Another early parenting intervention consisted of 2 psychoeducational group sessions and 4 individual sessions [123]. The group sessions provided information about the typical characteristics of preterm infants, their special caregiving needs, and the procedures conducted by NICU staff. This information was delivered by a pediatric neuropsychiatrist and a neurodevelopmental therapist. The individual sessions consisted of the joint observation of the infant and communication skills to support parenting efforts. When assessing psychomotor development using the Griffith Mental Development Scales at a CA of 24 months, the intervention group demonstrated significantly higher global scores and hearing-speech scores than the control group [123].
Family-centered care
Considering the previously discussed content, it becomes evident that promoting breastfeeding, increasing parent-infant interactions in an SFR setting, encouraging parental book reading and language interventions, and facilitating parent-integrated interventions in the NICU may enhance the language development of preterm infants. All of these strategies can be implemented through family-centered care.
Family-centered care enables parents to act as the primary caregivers of their babies and partners with clinical teams. Key concepts of family-centered care include an environment that is designed to support 24-hour parental presence/participation, NICU team education and support, parent education/psychological support, and active parent participation/partnership [124,125]. There is growing evidence of the potential advantages of family-centered care of hospitalized preterm infants. Additionally, it enhances breastfeeding rates and contributes to increased weight gain [126-128]. Moreover, it reduces morbidities such as retinopathy of prematurity, bronchopulmonary dysplasia, infections, and intraventricular hemorrhage, leading to a decreased length of hospital stay. 126,127)Ultimately, family-centered care reduces parental stress and anxiety and improves neurodevelopmental outcomes, including language development [126,127,129,130].
Despite its benefits, implementing family-centered care in the traditional NICU is challenging due to difficulty changing the perceptions of healthcare professionals and parents, a shortage of well-trained specialists, and issues related to sustained fidelity. As a result, family-centered care is still not implemented in most NICU facilities in Korea. To implement family-centered care, a fundamental shift in mindset is required that moves away from professional-centered care and hierarchical hospital culture. Parents must be considered the primary caregivers and recognized as collaborative partners in all aspects of NICU care. There is also a need to develop early assessments and systematically tailored intervention programs to implement family-centered care plans. Adopting a multidisciplinary team approach, including speech therapists, otolaryngologists, neurologists, and rehabilitation medicine specialists, is crucial. This approach should also encompass parental education regarding language development and intervention along with programs aimed at providing emotional support to parents. Ultimately, it is crucial to develop standardized protocols and national policies, establish standardized assessment metrics, and ensure national financial support for the successful integration of family-centered care into the domestic health care system.
Conclusions
Preterm infants often face speech and language developmental delays of multifactorial etiologies. Promoting breastfeeding, increasing parent-infant interactions in an SFR setting, encouraging a nurturing language environment that consists of parental book reading and language interventions, and facilitating parent-integrated interventions in the NICU may enhance the language development of preterm infants. These supportive strategies can be integrated through family-centered care, which recognizes parents as primary caregivers and collaborative partners. These strategies for language development implemented in the NICU are expected to encourage a positive parent-child relationship and sustained language development efforts even after discharge.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: JSH, EKK; Data curation: JSH; Formal analysis: JSH; Methodology: JSH, EKK; Project administration: JSH, EKK; Visualization: JSH; Writing-original draft: JSH; Writing- review & editing: JSH, EKK