Outcome of pediatric inflammatory bowel disease in Asian children: a multinational 1-year follow-up study
Article information
Abstract
Background
Epidemiological data on pediatric inflammatory bowel disease (PIBD) have been reported in Asian countries. However, short-term follow-up data, especially in Southeast Asian countries, are limited.
Purpose
Analyze and compare the baseline and 1-year follow-up (1FU) data for PIBD in Asian children.
Methods
The multinational network included patients with PIBD (aged <19 years) in 5 Asian countries (Malaysia, Philippines, Singapore, Sri Lanka, and Thailand). The diagnosis of PIBD requires gastrointestinal endoscopy. The patients' demographics, clinical information, disease- related outcomes, and treatment data at 1FU were collected.
Results
In 1995–2021, 368 patients were enrolled (Crohn disease [CD], 56.8%; ulcerative colitis [UC], 38%; and inflammatory bowel disease [IBD]-unclassified, 5.2%). At 1FU, symptoms including diarrhea, bloody stools, and nausea/vomiting subsided in <3%, while abdominal pain persisted in 10.5% of patients with CD and 7.1% of patients with UC. Assessment endoscopy was performed at 1FU in 38% of CD and 31% of UC cases, of which 21% and 23% showed mucosal healing, respectively. Oral prednisolone was administered to 55.3% of patients at diagnosis and 26.8% at 1FU, while infliximab was administered to 2.5% and 7.2% of patients at diagnosis and 1FU, respectively. Independent factors of 1-year clinical remission for CD were oral prednisolone (odds ratio [OR], 0.20; 95% confidence interval [CI], 0.06–0.68), antibiotic use (OR, 0.09; 95% CI, 0.01–0.54), and immunomodulator use (OR, 5.26; 95% CI, 1.52–18.22). A history of weight loss at diagnosis was the only independent risk factor of an IBD flare by 1FU (OR, 2.01; 95% CI, 1.12–3.63).
Conclusion
The proportion of children with PIBD and abdominal pain at 1FU remained high. The rates of repeat endoscopy and infliximab use were suboptimal with high rates of systemic corticosteroid use. Quality improvement based on the aforementioned predictors may enhance PIBD care in this geographic region or similar settings.
Key message
Question: Short-term (1-year) follow-up data in pediatric patients with inflammatory bowel disease (IBD), especially in Southeast Asian countries, are limited.
Finding/Meaning: Abdominal pain and pallor rates remained high at 1 year after IBD diagnosis. Three independent factors of 1-year clinical remission for Crohn disease were oral prednisolone, antibiotic, and immunomodulator use at 1-year follow-up. A history of weight loss at diagnosis was the only independent risk factor of IBD flare.
Introduction
Pediatric inflammatory bowel disease (PIBD) presents up to 25% of all incident cases of inflammatory bowel disease (IBD) [1]. The disease can be divided into 3 subtypes: Crohn disease (CD), ulcerative colitis (UC) and inflammatory bowel disease-unclassified (IBD-U) [2]. Systematic reviews reported a rising prevalence among children residing in most regions in the world, including many newly industrialized countries in Asia [3,4]. Data from the multinational collaborative studies to define the characteristics and courses of PIBD children in the Asian countries including Malaysia, Philippines, Singapore, Sri Lanka, Taiwan, and Thailand have been reported [5].
PIBD usually presents with chronic and/or bloody diarrhea, abdominal pain, weight loss, fever, and various extraintestinal manifestations in conjunction with history of other pre-existing immune-related diseases [6]. Besides the initial blood and stool tests, the diagnosis requires endoscopy (i.e., mostly upper gastrointestinal [GI] endoscopy and colonoscopy) and mucosal biopsy. Once the diagnosis is confirmed, therapeutic choices are made based on the disease phenotype (CD or UC), classification, and disease behavior. Conventional management include exclusive enteral nutrition (EEN), systemic corticosteroids, 5-aminosalicylic acid (5-ASA), immunomodulators such as azathioprine and methotrexate, and biologic agents such as infliximab and adalimumab [7]. Bowel inflammation in most patients with PIBD can be controlled by the aforementioned management, but the disease can flare up at any time after diagnosis, and the factors of disease flare vary across studies [8-10]. While clinical remission may be one of the conventional targets after treatment initiation, mucosal healing (MH) has recently become the new target for disease control [11].
Short-term outcome on resolution of signs/symptoms, disease activity, medications, and disease-related outcomes in PIBD as well as predictive factors for each outcome from this region is limited. We therefore aimed to analyze and compare the baseline and 1-year follow-up (1FU) outcome in the aforementioned aspects.
Methods
1. Multicenter Asian PIBD registry network
The multinational collaborative network (called Asian PIBD Multicenter Registry Network) was initiated in 2017 to include 6 Asian pediatric gastroenterology centers from 5 South and Southeast Asian countries (Malaysia, the Philippines, Singapore, Sri Lanka, and Thailand) with the aim to study the characteristics, clinical practice, and health outcomes of PIBD in this region. All institutions are tertiary care teaching hospitals and have university-based settings with referrals from the hospitals within the area. Data for patients diagnosed with IBD before the age of 19 years from the participating centers were collected anonymously. IBD diagnosis was made based on endoscopy and histology. Data for children diagnosed prior to 31st December 2017 was collected retrospectively, and data for children diagnosed from January 2018 was collected prospectively. Medical tourists were excluded.
2. Definitions and data collection
The diagnosis and classification of PIBD into one of 3 disease subtypes (CD, UC, IBD-U) were determined by each participating center based on the revised Porto criteria [2]. Diagnosis of PIBD, disease phenotype and behavior was classified according to Paris modification of Montreal classification into CD, UC, and IBD-U [12]. Disease severity was defined based on the Pediatric Crohn’s Disease Activity Index (PCDAI) (inactive: <10, mild: 10–<30, moderate: 30–<40, severe: ≥40) [13] and Pediatric Ulcerative Colitis Activity Index (PUCAI) (inactive: <10, mild: 10–<35, moderate: 35–<65, severe: ≥65) [14]. Clinical remission was defined if either PCDAI or PUCAI was <10.
A perianal manifestation was defined if large perianal tags, fistula and/or abscess or collection were noted. Perianal involvement was defined as the presence of fistula (including fistula diagnosed with imaging studies), anal canal ulcers or abscesses [12], as well as asymptomatic or inflamed perianal tags and fissures. Data collected included patient demographics, diagnostic evaluation and disease characteristics based on clinical examination, laboratory, endoscopy and radiology investigations were collected. Information on the management, medication, and disease outcomes such as 1-year clinical remission and systemic corticosteroid use were also collected. Disease flare and MH was defined by clinicians at each institution independently. At 1FU, we accounted for the data within the range of 3 months (i.e., 9–15 months since the PIBD diagnosis).
3. Biostatistical analyses
The analyses were performed using Stata 18.0 (StataCorp LLC, College Station, TX, USA). Study variables were presented as frequency and percentage, mean with standard deviation (SD), or median with interquartile range. Categorical variables were compared using a t test, Mann-Whitney U test, chi-square test, or Fisher exact test. For the multiple category levels such as disease severity, we also applied marginal homogeneity for comparison. Univariate and multivariate analyses were performed using binary and multiple logistic regression for the interested factors. Forward stepwise selection was performed to choose the variables with P<0.10 to enter the multivariable models. Statistical significance was defined as P value <0.05.
4. Ethics approval
Study data were collected and managed using REDCap electronic data capture tools hosted at the Singapore Clinical Research Institute. Institutional Review Boards/Ethics Committees of each institution approved the study with formal data transfer agreements between institutions.
Results
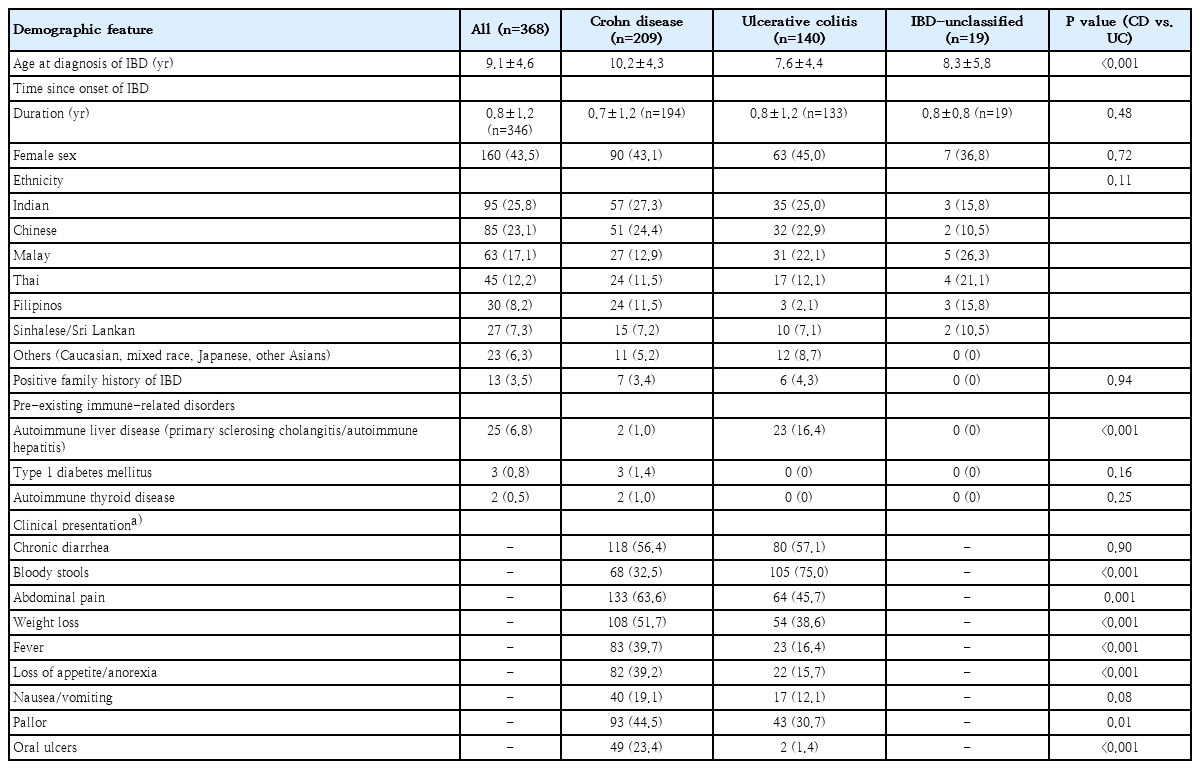
Over the period of 1995–2021, 368 cases were included in the database with a diagnosis of CD in 56.8%, UC in 38.0% and IBD-U in 5.2%. Retrospective dataset comprised 59% of the study population. The mean age at diagnosis was 9.1 years (SD, 4.6) with UC being diagnosed at a younger age (7.6 years) vs. CD (10.2 years) (P<0.001). Time to diagnosis (i.e., duration from disease onset to PIBD diagnosis) was approximately 9 months for all patients. Baseline epidemiological data for all patients is shown in Table 1.
1. Symptom and clinical/laboratory profiles at diagnosis
The main clinical presentation symptoms in our patient cohort are also shown in Table 1, with significant differences between children with CD versus UC. Abdominal pain, weight loss, fever, loss of appetite, pallor, and oral ulcers were more pronounced in CD. Perianal involvement was noted in 35% of CD cases with indolent fistula, draining fistula or abscess documented in 14%. On the other hand, bloody diarrhea was more common in UC (75% vs. 32.5% in CD).
Among CD, ileocolonic (L3) was the most common disease involvement (40.7%), while 35% had an upper GI involvement. Most (77%) CD children initially presented with inflammatory behavior (B1). For UC, 63% had pancolitis (E4) at the presentation but 65% had never had severe disease (S0). Disease phenotype for CD and UC is shown in Table 2.
The mean hemoglobin and erythrocyte sedimentation rate were 10.7 g/dL and 46 mm/hr without significant differences between CD and UC; while C-reactive protein was higher (51.6 mg/L vs. 20.6 mg/L) and serum albumin level was lower (32.5 g/L vs. 35.3 g/L) in CD when compared with UC children, respectively. Data on fecal calprotectin in this retrospective cohort is limited.
2. Comparison between the baseline and 1FU data
1) Signs and Symptoms at 1FU
Most pertinent symptoms such as diarrhea, bloody stools, vomiting, poor appetite, and vomiting subsided with the rates between 1.95%–2.9%, while abdominal pain remained 10.5% in CD and 7.1% in UC, and pallor and abdominal tenderness on examination still persisted at approximately 5% and 3% for both CD and UC children, respectively.
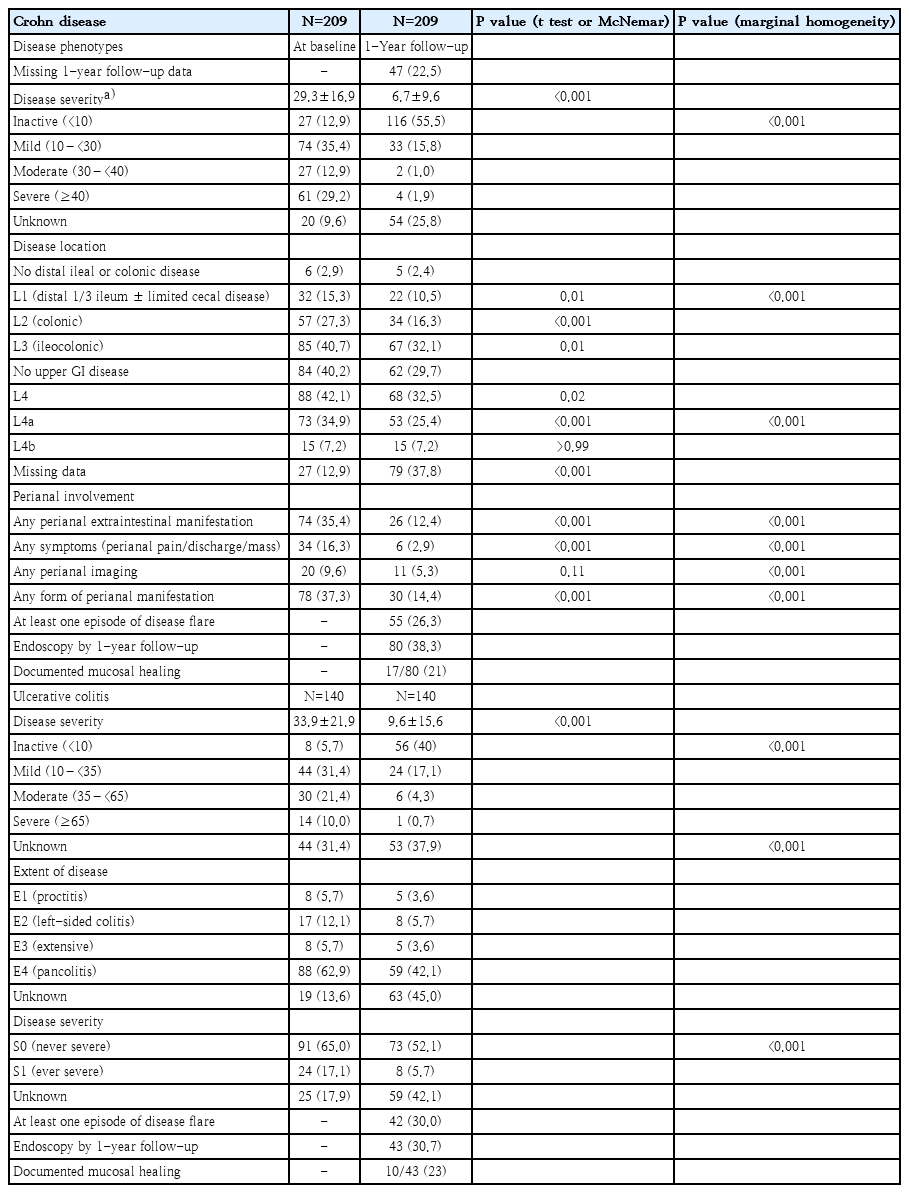
2) Disease severity and disease-related outcomes (Table 2)
For CD, 48.3% had inactive or mild disease and 29.2% had severe disease at diagnosis with a mean PCDAI of 29.3, while 55.5% achieved clinical remission (PCDAI <10) by 1FU. For UC, 37.1% had inactive and mild disease and only 10% had severe disease at presentation with a mean PUCAI of 33.9, while 40% achieved clinical remission. P values comparing the mean disease activity score and disease severity category were <0.0001 for the t test and testing for marginal homogeneity, respectively for both phenotypes. Approximately one-quarter (26.3%) of children with CD and 30% of UC had at least one flare episode within 1FU, respectively. Among CD, 38% underwent assessment endoscopy at 1FU and 17 of 80 (21%) had documented MH. While UC, 31% underwent endoscopy and 10 of 43 (23%) had MH. The overall MH rate was 22% at 1FU.
3) Medical management
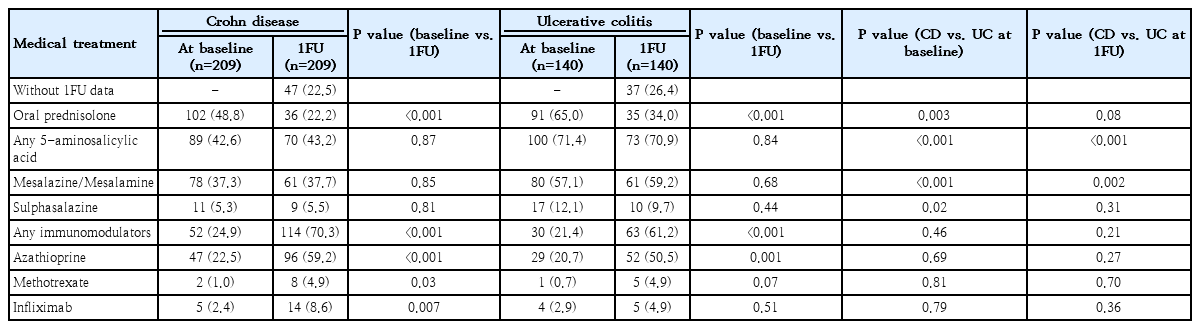
EEN induced remission in 87 of 209 (41.6%) of CD children, mainly in 2 countries (i.e., Malaysia and Singapore). Most received EEN for at least 6 weeks (6–8 weeks in 49% and >8 weeks in another 28%) while 20% also had EEN prescribed at the 1FU timepoint.
Comparing management between 1FU versus baseline (Table 3), oral prednisolone was given in 55.3% at diagnosis and 26.8% at 1FU. Any 5-ASAs were given in 54.2% at diagnosis and 54.0% at 1FU. Any immunomodulators were given in 23.5% at diagnosis and more commonly given at 1FU (66.8%, P<0.001) with an increase of almost three-folds for both CD and UC. Infliximab was given only in a minority of children (2.5% at diagnosis and 7.2% at 1FU).
When comparing CD versus UC, almost two-thirds of the children with UC had oral prednisolone at diagnosis and approximately one-third were still receiving it at 1FU, while 5-ASAs were more commonly used both at diagnosis and 1FU in UC children. Immunomodulators and infliximab use were comparable between the 2 PIBD phenotypes at both time points.
3. Multivariable analyses of the interested disease-related outcomes
To determine the associated factors with various disease- related outcomes such as clinical remission, disease flare, and MH; multivariable logistic regression analyses were performed for the baseline and clinical characteristics at diagnosis as well as the treatment at both time points (at diagnosis and 1FU).
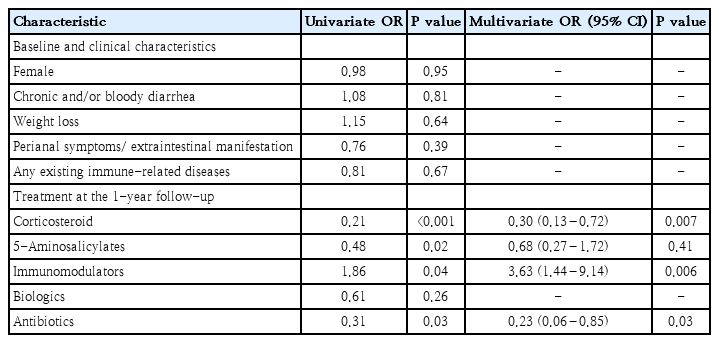
1) One-year clinical remission
The baseline demographics and clinical characteristics such as gender, chronic and/or bloody diarrhea, weight loss, or any pre-existing immune-related diseases as well as treatment at baseline (i.e., corticosteroid, 5-ASA, immunomodulators) were not statistically significant in the univariable analyses. The use of oral prednisolone, 5-ASA, and antibiotics at the 1FU were less likely to achieve clinical remission, while the use of immunomodulators was more likely to achieve clinical remission (all P<0.05). The use of biologics was not significantly different either at baseline or at 1FU (P=0.06 and P=0.26, respectively). In the multivariable analyses, oral prednisolone (odds ratio [OR], 0.30; 95% confidence interval [CI], 0.13–0.72), antibiotics (OR, 0.23; 95% CI, 0.06–0.85), and immunomodulators (OR, 3.63; 95% CI, 1.44–9.14) remained as significant independent factors of 1-year clinical remission (Table 4).
Stratifying by the PIBD phenotype, the independent factors remained relatively similar for CD (the use of oral prednisolone [OR, 0.20; 95% CI, 0.06–0.68], antibiotics [OR, 0.09; 95% CI, 0.01–0.54], and immunomodulators [OR, 5.26; 95% CI, 1.52–18.22] at the 1FU). However, for UC, a single predictor of 1-year clinical remission was using 5-ASA at diagnosis (OR, 17.40; 95% CI, 1.57–193).
2) Disease flare within 1 year after PIBD diagnosis
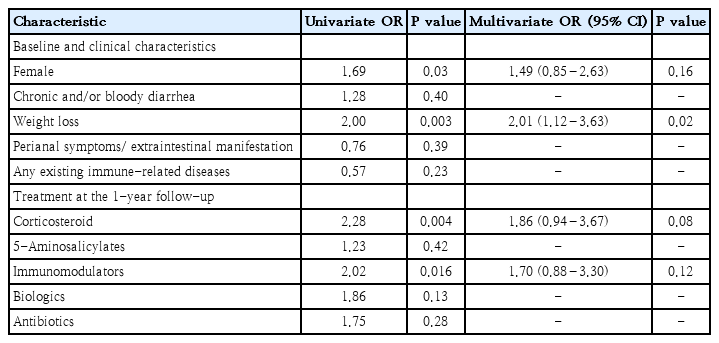
Female gender and history of weight loss at diagnosis posed a higher chance of disease flare in the univariable analysis. Oral prednisolone and immunomodulators at baseline 1FU were also 2 other significant factors in the univariable analyses but not in the final multivariable analysis (Table 5). The only independent factor of disease flare was history of weight loss at diagnosis (OR, 2.01; 95% CI, 1.12–3.63). When stratifying for the PIBD phenotype, female gender (OR, 2.27; 95% CI, 1.04–4.76) and history of weight loss (OR, 2.73; 95% CI, 1.26–5.91) remained significant predictors in CD, but neither factors were significant for UC.
3) Mucosal healing
Among 27 cases who reached MH (17 in CD and 10 in UC) in 123 children that underwent repeated endoscopy at 1FU, none of the baseline and treatment-related variables demonstrated statistical significance in the univariate analyses therefore multivariable analyses were not subsequently performed. The relatively small number of children in each of the 2 PIBD phenotypes who reached MH limited further analyses.
Discussion
This multicenter international registry among mainly Southeast Asian countries demonstrated several aspects in PIBD that either similar or differ from the existing literature mostly conducted in the Western countries such as a younger age at PIBD diagnosis, comparable proportions of significant perianal disease and clinical remission, and a higher rate of steroid dependency at 1FU in this region. Among 368 PIBD patients, we found that the mean age at diagnosis was approximately 9 years with UC being diagnosed at an earlier age when compared to CD. A recent multinational Asian PIBD cohort in 2022 reported the comprehensive epidemiological data elsewhere; the similar cohort to this current report [5]. The average age at UC diagnosis in this cohort was relatively similar to a cohort of Thai children (7.6 and 7.8 years) [15]. In a recent German registry, the median age at diagnosis of CD was approximately 12 years, which was almost 2 years older than CD children in this cohort [16]. Furthermore, in a cohort of 200 Indian children, the median age of diagnosis of PIBD was 15.3 years [17]. An earlier (younger) onset of the disease in the Southeast Asian region may be explained by multifactorial factors such as ethnicity and genetic background as well as different environmental factors such as disrupted gut microbiota [18] or GI infections [19]. Besides abdominal pain, systemic manifestations (i.e., weight loss, fever, anorexia, pallor on examination) were more common in CD when compared to UC, while bloody diarrhea was more common in UC; the findings that were similar to the previous reports in Southeast Asia and other regions [5,7,15]. The proportion of perianal disease with indolent fistula, draining fistula or abscess in this cohort (14%) was comparable to the children residing in the West (10%–13%) [20-22], but much lower than the proportion in Japanese children [23]. The findings on higher C-reactive protein and lower serum albumin in CD (vs. UC) are consistent with a previous report [15].
This study highlighted the 1FU data after the initial PIBD diagnosis with regards to clinical data, disease activity, disease-related outcomes, management as well as multivariate analyses on various disease-related outcomes. We found that the most pertinent symptoms subsided by 1FU but some subjective symptoms/signs such as abdominal pain (10.5% in CD and 7.1% in UC) or pallor on examination were more likely to persist as compared to other symptoms such as diarrhea, bloody stools, or vomiting. Reviews have shown that IBD patients with clinical remission can present with diarrhea-predominant irritable bowel syndrome-like symptoms which has been associated with mucosal inflammation and increased permeability, and possibly with dysregulated gut-brain interactions [24]. However, abdominal pain at the 1FU could also be a signal of bowel inflammation and disease non-remission even without obvious diarrhea or bloody stools. In a survey-based study of 122 IBD adults with clinical remission in the UK, 27% had chronic abdominal pain, and adults with pain had higher scores in various pain-related domains such as somatization, highly sensitive person scale, and coping resources inventory health [25]. Even abdominal pain is reported with a higher proportion when compared to other symptoms at 1FU, the younger age of patients may limit the report of subjective symptoms such as abdominal pain.
Even though the disease activity scores decreased over time, the clinical remission rates were not high at 55.5% in CD and 40% in UC at 1FU. A recent study from Israel enrolled cases treated with thiopurines without biologics and found that steroid-free clinical remission at 1FU was 39% in both CD and UC [26], while a study from the UK in 2014 showed a 1FU remission rate of 65% among CD children taking azathioprine [27]. We believed that the steroid-free clinical remission in our PIBD children would likely be much lower than the aforementioned rates due to a high rate (26.8%) of systemic corticosteroid use at 1FU. Disease flares of at least one episode were also common (26.3% and 30% for CD and UC, respectively), the rate was lower than a cohort from Croatia (41.2% in CD and 53.9% in UC, respectively) [28]. The rates of repeated endoscopy were relatively suboptimal (38% and 21% for CD and UC, respectively), and MH was noted only in 22% of patients who underwent repeated endoscopy at 1FU. We believed that the low MH rate was likely because (1) the disease was initially severe.; (2) the treatment may be suboptimal or inadequate to control the intestinal inflammation.; and/or (3) a small minority of children had endoscopic assessment at 1 year after diagnosis. These children probably also had symptoms or biochemical abnormalities at time of endoscopy. Studies and recommendations have shown that MH is associated with better health-related outcomes in the long term [11]. We therefore encourage health care personnel in this region or similar settings to consider repeating endoscopy after initiating the treatment, despite of clinical or biochemical remission.
For medical management, a high proportion (55.3%) of PIBD children received systemic corticosteroid at diagnosis and still received it in 26.8% at 1FU, while infliximab was given in a small percentage (<10%) of patients (Table 3). National data from Israel and Hungary reported steroid dependency at 1 year after UC diagnosis of 10% and 13%, respectively [22,29] which was much lower than our cohort (34% in UC). For that reason, we believe the patients who failed to enter remission and/or had recently flared by 1FU did not receive efficacious agents such as biologics, therefore the bowel inflammation was not well-controlled. When comparing with CD, both oral prednisolone and 5-ASA were more commonly used in UC at diagnosis; while at 1FU, 5-ASA remained more commonly used among UC. Aminosalicylates have been the main maintenance agent for UC children especially cases with mild to moderate disease [30]. Interestingly, 42.6% and 33.5% of CD children in our cohort were given 5-ASA at diagnosis and 1FU, respectively. According to the PIBD Working Group of Asian Pan-Pacific Society for Paediatric Gastroenterology, Hepatology, and Nutrition, 5-ASA may be considered as induction therapy in mild CD colitis in resource-limited settings and if EEN is not available or when EEN is not acceptable [31], this scenario may apply to some of the study population. EEN has been mainly used in Malaysia and Singapore but not the other 3 countries in this registry due to a limited availability at a national level. The aforementioned Working Group recommended azathioprine (i.e., immunomodulator) as a first-line treatment for CD maintenance similar to the ECCO-ESPGHAN guideline [32], which would match with an increase of almost three-fold of immunomodulator use at 1FU when compared to the time of diagnosis, the trend that is relatively similar to a previous study in CD children [22].
Treatment-related variables at the 1FU such as the use of oral prednisolone, antibiotics, and immunomodulators have been shown to be associated with a 1-year clinical remission (Table 4). Oral prednisolone and antibiotics were both associated with a lower chance of achieving remission while immunomodulator use was associated with a higher chance of remission, mainly for CD, but not for UC. None of the baseline or clinical data were associated with clinical remission.
Oral antibiotics as induction or maintenance therapy for both CD and UC are not recommended except for a short-term therapy in selected children with acute severe colitis [31]. Using antibiotics within 1FU may pose a risk of failure to reach clinical remission and a higher risk of relapse according to a population-based study from Denmark [9].
Using 5-ASA at diagnosis was associated with a 1-year clinical remission among UC. It is well known that 5-ASA is the mainstay agent for UC that can be given from the time of diagnosis [30]. A recent study also demonstrated that 5-ASA decreased risk of complicated disease course in pediatric-onset UC [29]. Therefore, the early use of 5-ASA (and probably with an appropriate taper of the commonly used oral prednisolone in more severe cases in our cohort) may lead to a favorable clinical outcome in a short-term period among UC children.
With regards to disease flare, a history of weight loss at diagnosis was found to be associated with a two-fold increased risk of flare by 1FU (Table 5). Among CD, both being female and a history of weight loss at diagnosis were significant independent predictors. Children with weight loss may have significant inflammatory burden prior to the diagnosis, carry a hard-to-control disease, and lead to a higher chance of disease flare later on. Gender difference in flare in CD is difficult to explain by biological plausibility. Hormonal effects were less likely playing a major role as substantial proportion of CD patients in the cohort were within the pre-pubertal stage. In contrast to our finding, an adult study from Norway (n=197) found no gender difference with regards to a disease relapse of CD during the first year after diagnosis [33]. Further studies on the gender-related difference in disease control and bowel inflammation in children are warranted.
Our group is aware that the PIBD data in this geographic region is severely limited. We therefore believe that information from this multinational registry would stimulate disease awareness within the comparable healthcare settings. However, this study has several limitations. As with most retrospective studies, missing data was an important issue such as disease activity scores were incomplete at the 1FU (26% in CD and 38% in UC). The information on compliance of the prescribed medication was difficult to evaluate in this study type. Repeated endoscopy to evaluate and define MH was performed in just approximately one-third of patients therefore robust statistical analysis to define factors associated with MH was limited.
In conclusion, by the 1FU after PIBD diagnosis, even the most pertinent symptoms subsided but the proportion of children with abdominal pain remained relatively high. The rates of repeated endoscopy and infliximab use were suboptimal, and the rate of systemic corticosteroid use remained high. Several treatments were associated with 1-year clinical remission, mainly in CD. Moreover, history of weight loss before diagnosis was a significant predictor in disease flare by 1FU after diagnosis. Quality improvement based on the aforementioned independent variables may enhance the care of PIBD within the comparable health care settings.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
Pornthep Tanpowpong received a mid-career Career Development Grant from the Faculty of Medicine Ramathibodi Hospital.
Author contribution
Conceptualization: PT, ST, MA; Formal Analysis: YW; Investigation: PT, JH, KC, KM, AR, WH; Methodology: PT, YW; Project Administration: WSL, MMA; Writing – Original Draft: PT; Writing – Review & Editing: ST, JH, WSL, MMA