Differential roles of interleukin-6 and adrenomedullin in early diagnosis and mortality predictions in late-onset neonatal sepsis
Article information
Abstract
Background
Diagnosing and predicting neonatal sepsis is challenging because of its nonspecific symptoms, lack of diagnostic criteria consensus, and absence of early, sensitive, and specific diagnostic laboratory tests.
Purpose
To evaluate the diagnostic and prognostic potential of adrenomedullin (ADM), interleukin-6 (IL-6), and C-reactive protein (CRP) in late-onset neonatal sepsis (LOS).
Methods
We studied 53 neonates with culture-proven LOS by sampling at admission and on antibiotic treatment days 3 and 7. These data were compared with those of 22 healthy full-term controls sampled on day 3 before hospital discharge. Survivors and nonsurvivors in the sepsis group were analyzed separately.
Results
Coagulase-negative Staphylococcus was the most commonly detected pathogen. ADM (cutoff, 0.5 ng/mL) and CRP (cutoff, <5 mg/L) values aligned with manufacturer recommendations, while IL-6 levels (cutoff, 10 pg/mL) were higher than expected, likely due to labor stress. The median biomarker levels significantly distinguished neonates with sepsis from controls (P<0.0001) at all time points with ADM and IL-6 levels elevated at admission, indicating their potential as early diagnostic markers. CRP level was diagnostically useful starting on day 3. Prognostically, IL-6 (P<0.001) and ADM (P<0.05) differentiated survivors from nonsurvivors; however, only IL-6 consistently predicted mortality at all time points (area under the curve [AUC] >0.90). ADM and CRP levels showed poor prognostic value (AUC<0.70). ADM and IL-6 demonstrated strong diagnostic utility in early LOS, whereas CRP became relevant later. IL-6 was the only reliable biomarker for predicting mortality, supporting its integration into clinical protocols. Combining IL-6 with CRP may enhance early detection and management, potentially improving neonatal outcomes.
Conclusion
IL-6 is a robust biomarker for the early diagnosis and prognosis of LOS. Incorporating IL-6 into clinical practice with CRP could improve early neonatal LOS diagnosis and patient outcomes.
Key message
Question: Can adrenomedullin (ADM) or interleukin-6 (IL-6) detect late-onset neonatal sepsis (LOS) at admission (area under the curve [AUC]>0.90) as an early diagnostic marker?
Finding: Only IL-6 consistently distinguished survivors from nonsurvivors (AUC>0.90) on admission and antibiotic treatment days 3 and 7. C-reactive protein level identified infections from day 3 but failed to predict outcomes (AUC<0.70).
Meaning: IL-6 level can improve LOS diagnosis and prognosis.
Graphical abstract. Receiver operating characteristic curves and areas under the curve (AUC) generated at neonatal intensive care unit admission for adrenomedullin (ADM), interleukin (IL)-6, and C-reactive protein. The left panel (septic neonates vs. controls) shows the best early diagnostic biomarkers, with ADM and IL-6 showing an AUC >0.90 (excellent diagnostic accuracy). The right panel (survivors vs. nonsurvivors) evaluates early prognostic biomarkers, and only IL-6 achieved an AUC >0.90 (excellent prediction accuracy).
Introduction
Neonatal sepsis is a life-threatening health condition associated with an infection that can lead to complications like shock or organ failure. It is categorized into early-onset sepsis (EOS) occurring within 72 hours of birth, and late-onset sepsis (LOS) which appears afterward. Sepsis and preterm birth are major neonatal health challenges, contributing to nearly 50% of deaths in children under five. However, efforts to reduce neonatal sepsis-related mortality, especially in low- and middle-income countries, have lagged behind those for older children [1,2].
Although EOS is more common, LOS is quite significant, affecting 1.6% of full-term and 12%–50% of preterm infants. The global neonatal mortality rate is around 18% and more than a half of this contingent is due to LOS [1-4].
A lack of consensus on neonatal sepsis definition complicates diagnosis, leading to inconsistent criteria, antibiotic overuse, prolonged neonatal intensive care unit (NICU) stays, increasing hospital-acquired infections and antimicrobial resistance [4,5].
Neonatal sepsis symptoms are nonspecific, and the current gold standard, blood culture, has limitations due to low bacteremia levels which characterize sepsis in neonates, insufficient blood volumes, processing delays, and prior antibiotic use [4-8].
Diagnostic tests include complete blood counts and cultures of blood, urine, and cerebrospinal fluid. C-reactive protein (CRP), an acute-phase protein produced in the liver, has a half-life of 19 hours and is the most commonly used biomarker of inflammation/infection in clinical practice, although it has low sensitivity for early diagnosis due to its characteristic 3-day delay to respond after the onset of infections [9,10].
Other biomarkers, including procalcitonin, presepsin, and proinflammatory cytokines show varying diagnostic accuracy [11-15]. However, multiple tests may improve diagnosis and prognosis in the context of neonatal sepsis [16,17].
Proinflammatory cytokines such as interleukin (IL)-6, IL-1β, tumor necrosis factor-α, and IL-8 rise before CRP, with IL-6 playing a key role in inflammation and immune response [18,19]. IL-6 has been linked to multiorgan dysfunction and mortality, making it a reliable marker for predicting outcomes in septic adult patients [20,21]. Its relatively longer half-life of around 15 hours compared to other cytokines enhances its prognostic potential [22].
Adrenomedullin (ADM), an active peptide with vasodilatory and immune-modulating effects, has a very short half-life of only 20–22 minutes but shows promise in sepsis, although the inactive and more stable midregional-ADM has been preferred in some reports [23-25].
This study aimed to evaluate the kinetics of the active short half-life ADM, IL-6 and the routinely assessed CRP to identify the most reliable biomarker for diagnosing and predicting prognosis in neonatal LOS.
Methods
1. Study design
This multicenter prospective cohort study of full-term and preterm neonates followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for cohort studies. Demographic data and microorganisms identified in blood cultures were recorded. For coagulase-negative Staphylococci, 2 positive cultures were required to include neonates in the sepsis group. ADM, IL-6, and CRP levels were measured at 3 collection points: on NICU admission (D0), on day 3 (D3), and day 7 (D7) posthospitalization and antibiotic administration. Two groups were studied: septic and noninfected neonates.
2. Ethics
This research was approved by the Institutional Research Ethics Committee on July 10, 2022 in accordance with the resolution number 466 of 2012 from the National Health Council which deals with research carried out on human beings. Septic neonates were included only after the mothers received a detailed explanation of the study's objectives, as well as the number and volume of blood samples required for research. Participation required the mothers' consent, formalized by signing the Free and Informed Consent Form specifically designed for this research, whose content had been previously approved by the Institutional Ethics Committee.
3. Infected neonates
This group included neonates with blood culture-confirmed LOS, and symptoms onset occurring after 72 hours of life. At each collection point, blood cultures, life-support exams (per NICU protocol), and CRP tests were conducted. Additionally, 1 mL of blood was drawn for IL-6 and ADM testing.
4. Control group
The control group consisted of neonates born to mothers with uneventful pregnancies and deliveries, who received prenatal care and delivered vaginally. These full-term neonates were breastfed and had no complications during the first 3 days of life. On the third day, after maternal informed consent, 2 mL of blood were obtained for ADM, IL-6, and CRP testing.
5. Classification of neonates
Gestational age was estimated using the mother's last menstrual cycle and first prenatal ultrasound. Neonates born between 37 and 42 weeks were classified as full-term, those under 37 weeks as preterm, and those over 42 weeks as post-term. Birth weight adequacy was calculated using Intergrowth 21st standards [26].
6. Inclusion criteria
Neonates had to present positive blood cultures (BACT ALERT 3D, BioMérieux, Marcy-l'Étoile, France) and at least one sepsis symptom (e.g., fever, cardiovascular, respiratory, gastrointestinal, or neurological issues) alongside one abnormal laboratory result (e.g., elevated CRP, altered blood counts).
7. Exclusion criteria
Conditions that might affect clinical evaluation were excluded, such as surgical procedures, severe perinatal asphyxia identified through Apgar scores<4 at 5 and 10 minutes [27], metabolic abnormalities (e.g., hypoglycemia, hypocalcemia), congenital malformations, and congenital infections.
8. Routine tests
Blood counts were performed using automated systems (Bayer ADVIA 120 or Abbott CELL DYN 4000). Blood count abnormalities were defined as leukocytosis (>20,000/mm³), leukopenia (<5,000/mm³), neutrophilia (>13,000/mm³), neutropenia (<1,000/mm³), immature neutrophil index >0.2, and thrombocytopenia (<100,000/mm³) [28]. CRP was measured using immunoturbidimetry (Cobas CRP test, Roche Diagnostics, Basel, Switzerland) and reference values recommended for adults are<5 mg/L.
9. Interleukin 6
IL-6 levels were measured using a highly sensitive enzyme immunoassay kit (R&D Systems, Minneapolis, MN, USA). Samples were tested in duplicate, with a detection range of 0.078–10 pg/mL. For concentrations >10 pg/mL, samples were diluted, tested again and final concentrations calculated by multiplying by the dilution factor.
10. Adrenomedullin
ADM levels were measured in 50-μL serum samples, tested in duplicate, using the ADM (1–52) Human EIA Kit (Phoenix Pharmaceuticals Inc., Burlingame, CA, USA). The detection range was 0.01–100 ng/m L and recommended reference values for healthy adults<0.5 ng/mL.
11. Statistical analysis
The normality of IL-6, ADM, and CRP levels across the 3 collection points was assessed using the Kolmogorov-Smirnov test. Cutoff values were established from true positive (septic neonates) and true negative (control) cases. Receiver operating characteristic (ROC) curves were generated for each biomarker at all collection points. Prediction accuracy was classified as acceptable (area under the curve [AUC], 0.70–0.80), good (AUC, 0.80–0.90), or excellent (AUC>0.90) [29]. Statistical analysis was performed using the GraphPad Prism ver. 8.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results
The study included 53 neonates with blood culture-proven LOS and 22 full-term healthy controls. Table 1 summarizes the distribution of the septic neonates based on isolated microorganisms, sex, birth weight, gestational age (per Intergrowth 21st standards), and outcomes (survivor and nonsurvivors).
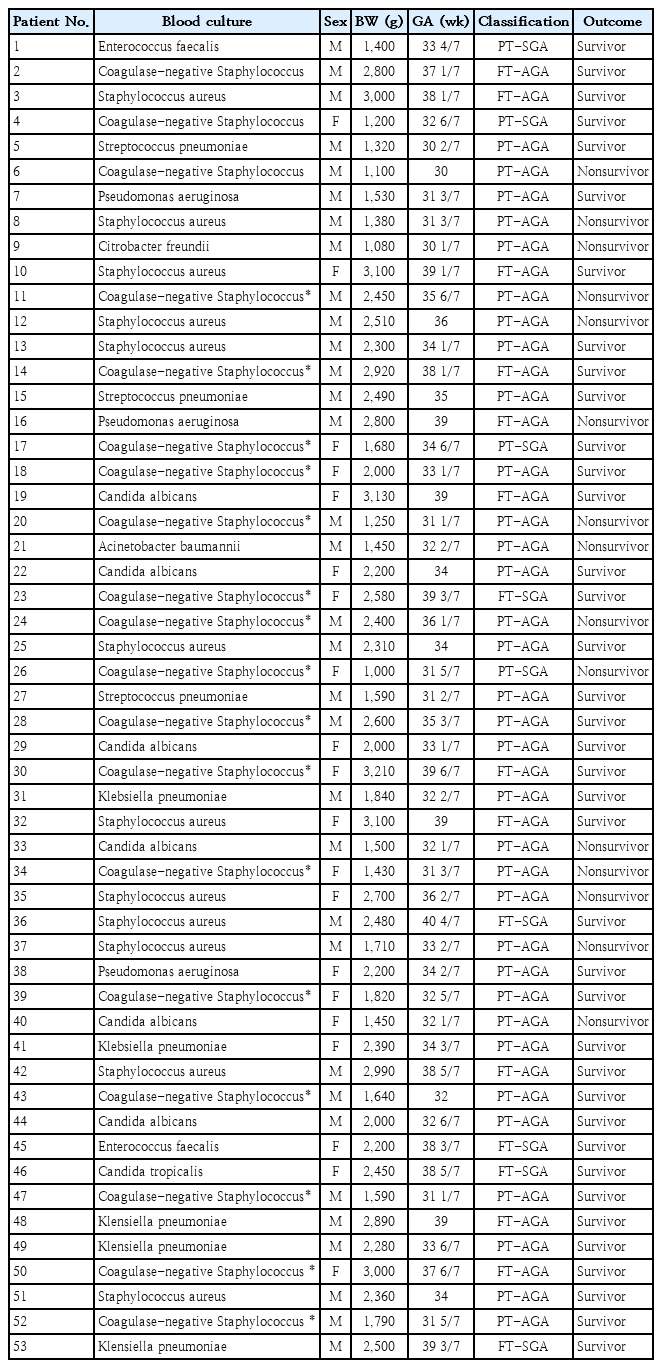
Distribution of 53 neonates with sepsis according to microorganisms isolated in blood cultures, sex, birth weight, gestational age, classification (intergrowth 21st) and outcome (survivor or nonsurvivor)
Among the 53 LOS cases, 33 were male (62.3%), with a median birth weight of 2,250 g (range, 1,000–3,210 g) and gestational ages between 30–40 4/7 weeks. Of these, 71.7% (38 of 53) were preterm, and 89.5% (34 of 38) were appropriate for gestational age (AGA). The most common pathogens were Gram-positive bacteria (64.2%), including coagulase-negative Staphylococcus (19 cases), Staphylococcus aureus (12 cases), and Streptococcus pneumoniae (3 cases). Gram-negative bacteria wre isolated in 12 cases (22.6% of infections), with 5 Klebsiella pneumoniae, 3 Pseudomonas aeruginosa, 2 Enterococcus faecalis, 1 Acinetobacter baumannii, and 1 Citrobacter freundii); Fungi were isolated in 7 cases (13.2% of infections), 6 Candida albicans, and 1 Candida tropicalis.
The mortality rate was 28.3% (15 of 53), with deaths primarily occurring in preterm, AGA boys (11 of 15). The median birth weight among nonsurvivors was 1,450 g (range, 1,000–2,800 g). Microorganisms isolated in the fatal cases included coagulase-negative Staphylococcus (n=6), Staphylococcus aureus (n=4), Candida albicans (n=2), Pseudomonas aeruginosa (n=1), Acinetobacter baumannii (n=1), and Citrobacter freundii (n=1).
The control group consisted of 22 full-term, AGA neonates (11 males and 11 females), all with birth weights >3,000 g and Apgar scores >7 at 5 and 10 minutes. These neonates were breastfed and discharged on the third day of life without complications.
ADM, IL-6, and CRP levels for both, septic and control neonates are presented in Supplementary materials 1–3, containing the statistical analysis of the overall cohort, in addition to an outcome analysis (survivors and nonsurvivors). Sensitivity, specificity, and predictive values for each biomarker at the 3 collection points (D0, D3, and D7) are shown in Table 2, and the best cutoff values were highlighted in bold.
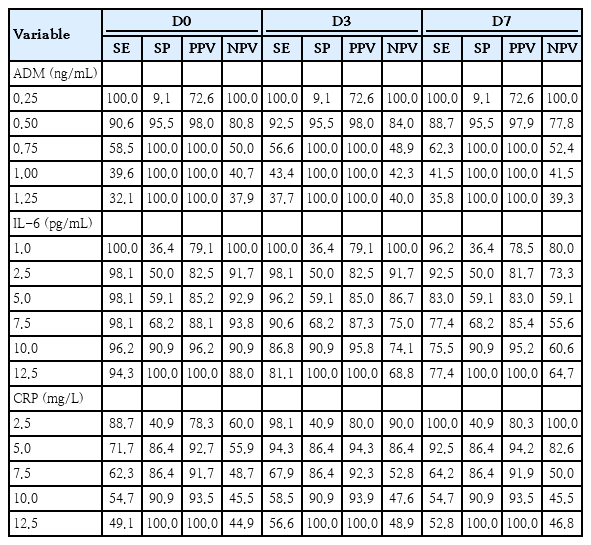
Cutoff values calculated of 3 biomarkers (ADM, IL-6, and CRP) in neonates with sepsis (n=53) and controls (n=22)
For ADM, the optimal cutoff was 0.5 ng/mL at all 3 collection points, consistent with the manufacturer recommendation. For IL-6, the best cutoff was 10 pg/mL, higher than the manufacturer’s reference value of around 1 pg/mL. CRP had a cutoff value<5 mg/L, also in line with the manufacturer guidelines (Table 2).
Table 3 compares median biomarker levels between septic neonates (n=53) and controls (n=22), showing that ADM, IL-6, and CRP could discriminate infected from noninfected neonates (Mann-Whitney test, P<0.0001) at all 3 collection points.
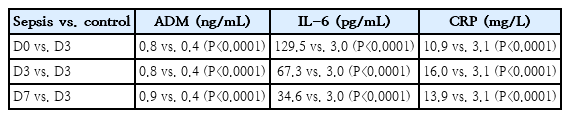
Median ADM, IL-6, and CRP levels at the 3 collection points (D0, D3, and D7) in the neonates with sepsis (n=53) versus only on D3 in the control group (n=22)
Table 4 shows the comparison between survivors (n=38) and nonsurvivors (n=15). CRP could not differentiate between the groups, while ADM showed significant differences at all collection points (D0, P=0.027; D3, P=0.046; D7, P=0.032), and IL-6 was the strongest predictor of all, with P<0.0001 at all collection points.
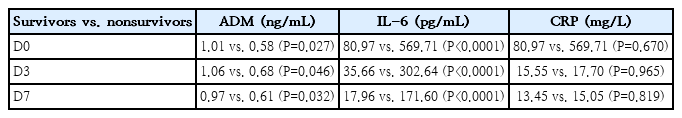
Median ADM, IL-6, and CRP levels at the 3 collection points (D0, D3, and D7), from the group of 38 survivors and 15 nonsurvivors (deaths)
1. Diagnostic value of ROC curves
ROC curves were generated to evaluate the diagnostic accuracy of biomarkers (Fig. 1). On D0, ADM had an AUC of 0.980 (95% confidence interval [CI], 0.956–1.000), IL-6 had an AUC of 0.985 (95% CI, 0.962–1.000), and CRP had an AUC of 0.805 (95% CI, 0.708–0.903). Both, ADM and IL-6 showed excellent diagnostic accuracy (AUC >0.90), while CRP did not.
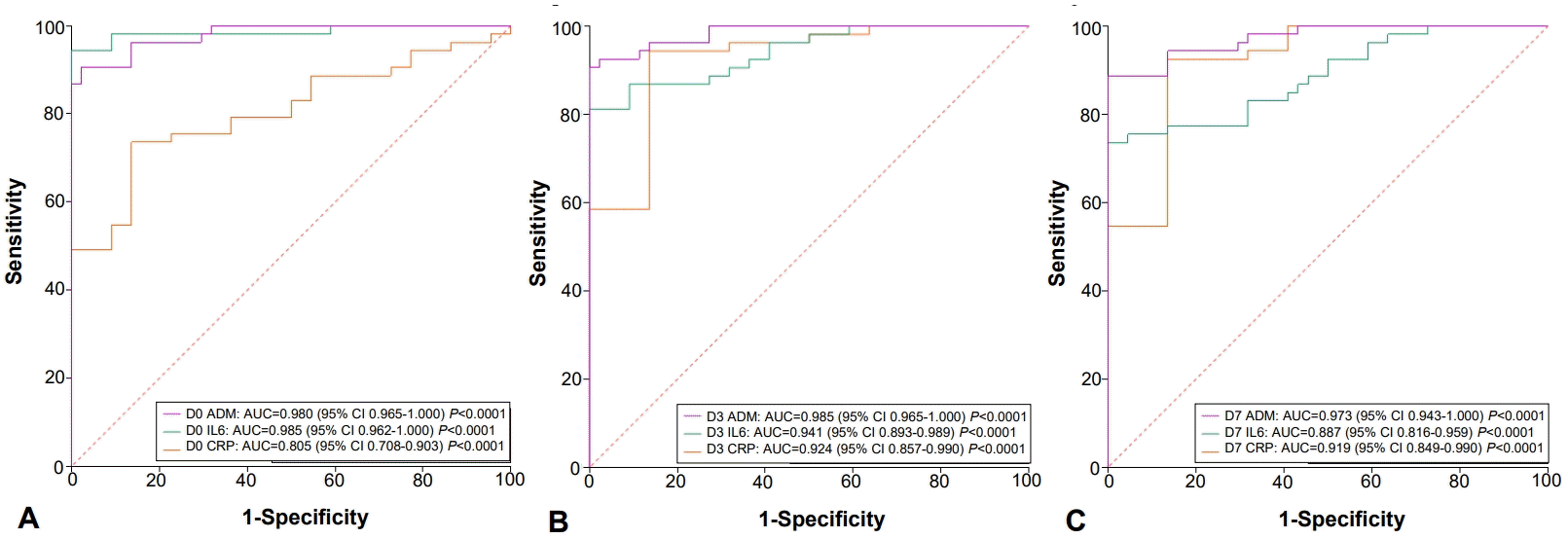
(A) Adrenomedullin (ADM), interleukin-6 (IL-6), and C-reactive protein (CRP) of sepsis cases versus controls on admission to the neonatal intensive care unit (day 0). (B) ADM, IL-6, and CRP levels of patients with sepsis versus controls on day 3 after hospitalization. (C) ADM, IL-6 and CRP of patients with sepsis versus controls on day 7 after hospitalization. D0, neonatal intensive care unit admission; D3 and D7, days 3 and 7 after hospitalization and prescription of antibiotics; CI, confidence interval.
On D3, ADM had an AUC of 0.985 (95% CI, 0.965–1.000), IL-6 had an AUC of 0.941 (95% CI, 0.893–0.989), and CRP had an AUC of 0.924 (95% CI, 0.857–0.990). The 3 biomarkers showed excellent diagnostic accuracy.
On D7, ADM had an AUC of 0.973 (95% CI,, 0.943–1.000), IL-6 had an AUC of 0.887 (95% CI, 0.816–0.959), and CRP had an AUC of 0.919 (95% CI, 0.849–0.990). At this collection point, IL-6 levels had decreased, while ADM and CRP remained elevated (AUC >0.90).
2. Prognostic value of ROC curves
ROC curves were also generated confronting survivors and nonsurvivors at the 3 collection points to evaluate the prognostic value of the 3 biomarkers, i.e., their ability in predicting outcomes (Fig. 2). On D0, ADM had an AUC of 0.696 (P=0.028), IL-6 had an AUC of 0.914 (P<0.0001), and CRP had an AUC of 0.535 (P=0.693). Only IL-6 showed excellent predictive accuracy (AUC >0.90).
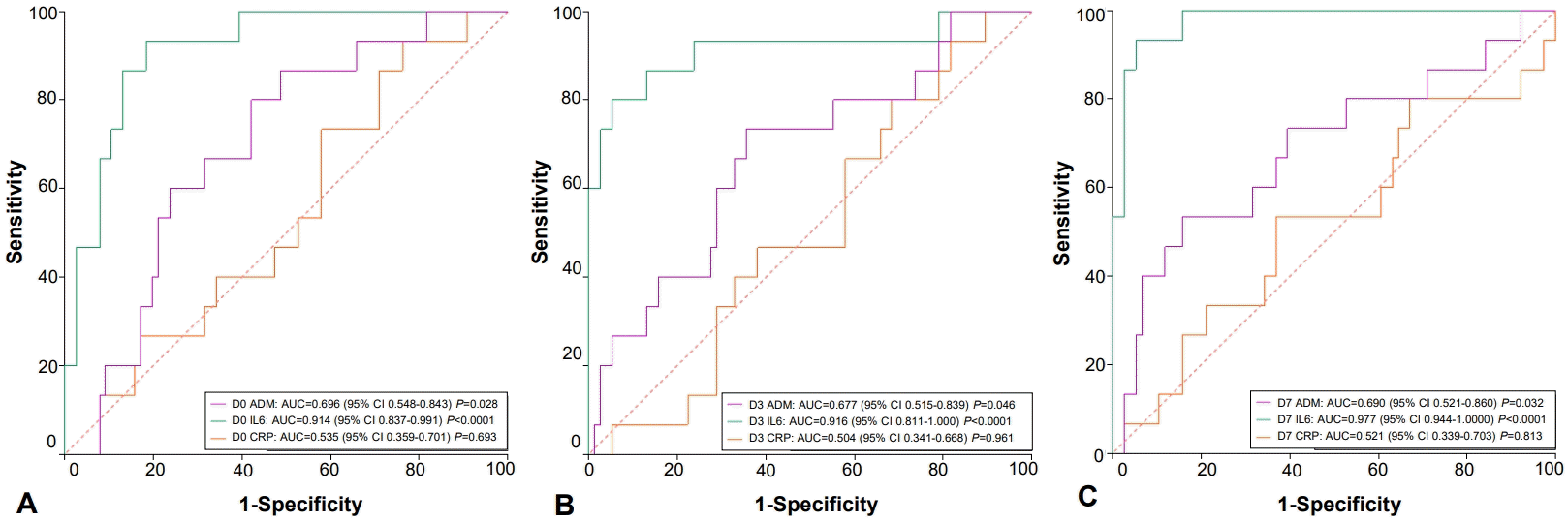
(A) Adrenomedullin (ADM), interleukin-6 (IL-6), and C-reactive protein (CRP) levels of survivors versus nonsurvivors on neonatal intensive care unit admission (day 0). (B) ADM, IL-6, and CRP levels of survivors versus nonsurvivors on day 3 after hospitalization. (C) ADM, IL-6, and CRP levels of survivors versus nonsurvivors on day 7 after hospitalization. D0, neonatal intensive care unit admission; D3 and D7, days 3 and 7 after hospitalization and prescription of antibiotics; CI, confidence interval.
On D3, ADM had an AUC of 0.677 (P=0.046), IL-6 had an AUC of 0.916 (P<0.0001), and CRP had an AUC of 0.504 (P=0.961). Again, only IL-6 accurately predicted outcomes.
On D7, ADM had an AUC of 0.690 (P=0.032), IL-6 had an AUC of 0.977 (P<0.0001), and CRP had an AUC of 0.521 (P=0.813). For the third time, IL-6 was the only accurate prognostic biomarker.
Discussion
This study aimed to monitor the kinetics of ADM, IL-6, and CRP from NICU admission through the 7th day of hospitalization and treatment, focusing on their diagnostic and prognostic potential in neonatal LOS. We compared 53 neonates with blood culture-proven sepsis (Table 1) to 22 full-term healthy controls born vaginally. Coagulase-negative Staphylococcus was the primary cause of LOS (Table 1), consistent with previous findings [30].
The cutoff values for ADM and CRP (Table 2) aligned with manufacturer recommendations (0.5 ng/mL and <5 mg/L, respectively), but IL-6 had a higher threshold (10 pg/mL). This increase is likely due to labor-induced stress, especially in vaginally delivered neonates, as reported by other studies [31,32].
Median biomarker levels on D0, D3, and D7 showed significant differences between septic neonates and controls (P<0.0001 at all 3 collection points) (Table 3). Both ADM and IL-6 demonstrated excellent early diagnostic value, with ROC curve AUC values exceeding 0.90 on admission (Fig. 1). ADM maintained consistent diagnostic reliability through day 7, while IL-6 performance declined after D3.
Previous studies have highlighted IL-6’s diagnostic potential when combined with CRP, IL-8, or other cytokines [11-18]. Our findings support IL-6 as a potent early diagnostic biomarker. While CRP could distinguish between infected and noninfected neonates (Table 3), but it did not perform as well diagnostically in ROC analysis (Fig. 1). Consistent with the literature, CRP levels showed a characteristic 3-day delay in reaching diagnostic levels [9,10,33], becoming diagnostic from D3 onward and persisting through D7. However, ADM emerged as a reliable early diagnostic marker throughout the entire 7-day period, contrasting with earlier findings that suggested biomarkers lose reliability 12–14 hours post-infection [15]. This underscores the value of combining biomarkers to improve diagnostic accuracy, as shown in studies of early-onset neonatal sepsis [16,34].
Regarding prognosis, IL-6 was the most reliable predictor of mortality, distinguishing survivors from nonsurvivors with P<0.0001 at all collection points (Table 4). ROC analysis confirmed this, with AUC values >0.90 on D0, D3, and D7 (Fig. 2). In respect to ADM and CRP, they also differentiated survivors from nonsurvivors (P<0.0001) (Table 4), but their prognostic power was weak, with AUC values<0.70 for both markers at the 3 collection points (Fig. 2).
Leal et al. [13] conducted a study examining 17 biomarkers (13 interleukins and 4 cytokines) in neonates at high risk for sepsis. Of the 96 neonates included, 50 (52%) were diagnosed with sepsis (26 EOS and 24 LOS). Elevated levels of IL-6, IL-10, and granulocyte colony-stimulating factor, along with reduced interferon-γ levels, were associated with sepsis development. Although IL-6 levels above 40.1 pg/mL were linked to sepsis and levels above 44.9 pg/mL to EOS, this study did not find IL-6 predictive of mortality in LOS, which contrasts with our findings.
A more recent study by Kurul et al. [35] assessed the relationship between IL-6, procalcitonin (PCT), CRP, and 7-day mortality in preterm infants with suspected LOS only at the time of infection suspicion. IL-6 and PCT levels were associated with mortality, with adjusted hazard ratios of 2.28 and 2.91, respectively (P<0.001), while CRP was not. This study did not analyze the progression of these biomarkers over time, as we did. In our study, we demonstrated that IL-6 and ADM were early diagnostic markers, and IL-6 accurately predicted mortality as early as admission. This predictive accuracy persisted on day 3 and continued through day 7, indicating that IL-6 is a robust predictor of mortality risk over the first week of hospitalization.
This study has some limitations, the most significant being the small sample size and the limited number of deaths in the case series. These constraints primarily arose from the additional blood volume required to measure the targeted biomarkers (IL-6 and ADM), which deterred participation. Of the 80 mothers approached, only 53 consented to the study and signed the informed consent form, resulting in a 34% loss of potential cases. This reduced the overall sample size and may have impacted the generalizability of the findings.
In conclusion, IL-6 consistently emerged as the most accurate biomarker for both early diagnosis and prognosis in neonatal LOS. ADM was a reliable diagnostic marker up to day 7, though it lacked prognostic value. CRP, while useful in certain diagnostic contexts, was confirmed as a late diagnostic marker with no prognostic significance. These findings underscore the importance of combining biomarkers with CRP and highlight IL-6 as the best candidate for enhancing diagnosis and outcome prediction in late-onset neonatal sepsis.
Supplementary material
Supplementary Tables 1-3 are available at https://doi.org/10.3345/cep.2024.01543.
Statistical analysis of ADM, IL-6, and CRP levels at the 3 collection points (D0, D3, and D7) in the group of 53 LOS cases and in the group of 22 controls
Statistical analysis of the three biomarkers (ADM, IL-6, and CRP) at the three collection points (D0, D3, and D7) in the group of 38 survivors
Statistical analysis of the three biomarkers (ADM, IL-6, and CRP) at the three collection points (D0, D3, and D7) in the group of 15 nonsurvivors (deaths)
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
Commercial ELISA kits, other reagents and disposable consumables for carrying out this research were funded by the São Paulo Research Foundation (FAPESP), process number 2021/04280-4, and this funding was granted to the to the Prof. Dr. Thelma Suely Okay - senior investigator. The first author (EHS) has been receiving a PhD scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), finance Code 001, Brazilian government, process number 88887.648600/2021-00. The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
We are thankful to FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for supporting this research project.
Author Contribution
Conceptualization: TSO; Data curation: TSO, EHS; Formal analysis: EHS; Funding acquisition: TSO; Methodology: EHS, GAB, MOS, MCPC, KAR, RA; Writing - original draft: TSO; Writing - review & editing: TSO, EHS.

