Serum bactericidal activity against meningococcus in patients with systemic lupus erythematosus
Article information
Abstract
Background
Patients with systemic lupus erythematosus (SLE) are susceptible to infectious diseases owing to various immunosuppressive treatments and disease characteristics. Meningococcal infections progress rapidly with a high incidence of severe complications and mortality; therefore, meningococcal vaccination is needed. However, there is limited evidence regarding the immunity and immunogenicity of patients with SLE.
Purpose
This study aimed to analyze the serum bactericidal activity against meningococci in patients with SLE in 2 domestic institutions in Korea.
Methods
Serum samples were collected from patients diagnosed with SLE (age <19 years) at Seoul National University Children's Hospital and Ewha Womans University Mokdong Hospital in 2016–2018. Serum bactericidal activity against the 4 meningococcal serogroups was analyzed using a serum bactericidal assay with rabbit serum. The patients' demographic information, diagnostic history, and disease activity status were obtained from electronic medical records.
Results
The mean age of the 41 included patients was 20.3±5.4 years (range, 10–35 years). All but one patient received steroids. The sera of most of the patients (34 of 41 [82.9%]) lacked bactericidal activity against serogroup A. Some patients showed bactericidal activity against serogroups C, W-135, and Y (63.4%, 56.1%, and 61.0%, respectively). There were no significant differences in the geometric mean indices based on complement consumption state or anti-double-stranded DNA antibody positivity.
Conclusion
Although the sera of some patients exhibited serum bactericidal activity against meningococci, most remained seronegative. It is important that patients with SLE at risk of meningococcal infection receive appropriate vaccinations. Our findings serve as baseline serological data for meningococcal vaccination policies for patients with SLE.
Key message
Question: What is the level of immunity against meningococcal infections in patients with systemic lupus erythematosus (SLE) under the age of 19, and is vaccination against meningococcus necessary for these patients, given their susceptibility to infections due to immunosuppressive treatments and disease characteristics?
Finding: Although some of our study patients exhibited serum bactericidal activity against meningococci, most remained seronegative.
Meaning: These findings suggest that patients with SLE who are at risk of meningococcal infection receive appropriate vaccinations.
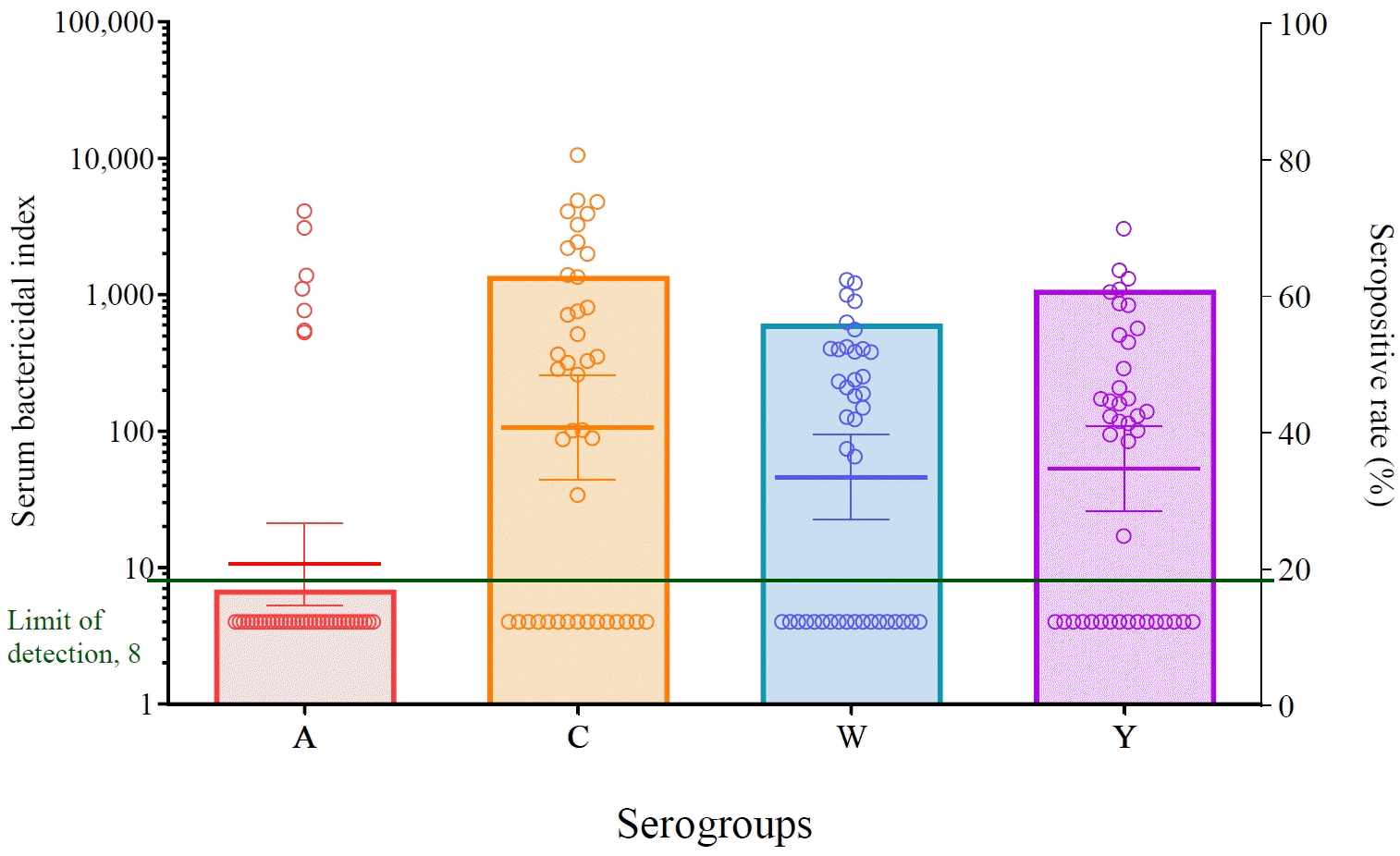
Graphical abstract. In this study, serum bactericidal activity against the 4 meningococcal serogroups was measured in adolescents and young adults with systemic lupus erythematosus (SLE). Scatter plot of the serum bactericidal indices (SBIs) and seropositivity rates for meningococcal serogroups in patients with SLE. Each dot represents the SBIs and the bar height indicates the seropositivity rate for each serogroup. Although some patients exhibited serum bactericidal activity against meningococci, most patients remained seronegative.
Introduction
Infection in patients with systemic lupus erythematosus (SLE) is a major cause of morbidity and mortality [1,2]. The protective immune function against bacteria, viruses, and fungi is impaired in patients with SLE, and they frequently develop opportunistic or serious infections. Serious infections are associated with mortality and occur in 11–45% of patients with SLE [3].
The lack of complements, defective pathogen phagocytosis, and inappropriately balanced levels of pro- and antiinflammatory cytokines are mainly responsible for the vulnerability to infection of patients with SLE [4,5]. Impaired immune activity cannot control the spread of pathogens and remove them, which results in persistent and worsening of infections. The manifestations and severity of SLE are dependent on the degree of uncontrolled autoimmune response or tissue invasion, which affects the defense immunity of patients.
Concomitant with intrinsic immune dysregulation, patients have an increased susceptibility to infection with respect to treatment. The use of immunosuppressive drugs, such as glucocorticoids (GCs), improves the prognosis of patients with SLE by preventing severe inflammation that either threatens their lives or causes organ damage. Although immunosuppressive therapy is effective and essential in controlling disease activity, it is associated with cardiovascular disease, diabetes, osteoporosis, and infection in patients with SLE [6,7]. Using GCs increases the infection risk of patients; however, some studies have reported no significant effects brought upon by GCs [8-12]. Hydroxychloroquine controls disease activity, prevents flare-ups or serious manifestations of the disease, including nephritis, and reduces serious infections in patients with SLE [3,13]. Several studies on the relationships between several immunosuppressive agents and infections in SLE have reported that using several immunosuppressive agents, including mycophenolate mofetil, azathioprine, cyclophosphamide, did not involve a significant risk of infection and mortality [9]. Given these inconsistent reports, there is a need for clinicians to obtain evidence from real-world clinical practice.
Meningococcal infections span a wide spectrum of clinical presentations, ranging from transient fever to severe shock, and the complement system is crucial in defending against meningococcal infections [14]. The complement profile of patients with SLE closely mimics that of inherited complement deficiencies; thus, immunoglobulin deficiency and defects in chemotaxis and phagocytic activity may contribute to the increased susceptibility of patients with SLE to meningococcal infections [14,15].
This study aimed to investigate the immunological status of meningococci in adolescent and young patients with SLE.
Methods
1. Participants and study design
Patients with childhood-onset SLE who visited a tertiary medical center between January 2016 and December 2018 were identified. The inclusion criteria were as follows: (1) fulfillment of the 1997 American College of Rheumatology revised classification criteria for SLE,16) (2) diagnosis before 19 years of age, and (3) treatment and follow-up at 2 tertiary medical centers (Ewha Womans Mokdong Hospital and Seoul National University Children’s Hospital). The exclusion criteria were as follows: (1) history of invasive meningococcal diseases, (2) received immunoglobulin within 3 months to one year before enrollment according to the dose and route, and (3) received antibiotics within 7 days before inclusion in the study. The medical records of these patients were retrospectively reviewed. The age of onset was defined as the age at the first clinical manifestation of SLE. Demographic characteristics, disease duration, and recent medications were also collected. Blood was drawn from each patient, and the complete blood count and inflammatory markers, including erythrocyte sedimentation rate, complement concentration, and anti-double stranded DNA antibody (anti-dsDNA Ab), were measured at enrollment.
2. Serum bactericidal assay for assessing immunogenicity
Serum bactericidal assays using baby rabbit complement (rSBAs) were performed for 4 meningococcal serogroups (A, C, W-135, and Y) at the Ewha Center for Vaccine Evaluation and Study as recommended by the World Health Organization in the standardized assay [17,18]. The target strains used for the rSBAs included ATCC13077 for serogroup A, C11 for serogroup C, MOI-240070 for serogroup W-135, and S-1975 for serogroup Y. ATCC13077 was purchased from the American Type Culture Collection (ATCC; Rockville, MD, USA); MOI-240070 was purchased from the National Collection of Type Cultures (Public Health England, Salisbury, UK); C11 was provided by GlaxoSmithKline Biologicals (Rixensart, Belgium); and Novartis Vaccines and Diagnostics (Cambridge, MA, USA). S-1975 was provided by the US Food and Drug Administration (Silver Spring, MD, USA).
All rSBA procedures were performed in a Class II biosafety cabinet. A frozen aliquot of the N. meningitidis strain from each serogroup was thawed and diluted in Hank's Balanced Salt Solution (Gibco, Carlsbad, CA, USA) containing 1% horse serum (HyClone, Logan, UT, USA) to yield 800–1,000 colony-forming units in 20 μL of solution. Duplicate serum samples were serially diluted threefold with buffer in 96-well U-bottom microtiter plates. Subsequently, 20 μL of meningococcal suspension was added to the serially diluted serum. After 2 minutes of incubation at 25°C with shaking at 700 rpm, 20 μL of baby rabbit complement (Pelfreeze, Brown Deer, WI, USA) was added to each well. The plates were incubated for 90 min (serogroup A) or 60 min (serogroups C, W-135, and Y) in a tissue culture incubator (37°C, 5% CO2) with shaking at 700 rpm. After stopping the reaction by placing the plates on ice for 15 min, we spotted 10 μL of the final reaction mixture onto 1.5% of Brain-Heart-Infusion (Becton Dickinson and Company, Sparks, MD, USA) agar plates with 5% horse serum. After the fluid was absorbed onto the agar, each plate was overlaid with molten Brain-Heart-Infusion agar (0.75%) containing 5% horse serum and 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO, USA). The plates were incubated at 37°C in a 5% CO2 incubator. We counted the surviving bacterial colonies in the plates using the colony counting software National Institute of Standards and Technology, US Integrated Colony Enumerator (Gaithersburg, MD, USA). We defined the bactericidal index as the serum dilution that killed 50% of the bacteria, as determined by linear interpolation. For computational purposes, an rSBA index (rSBI) lower than the serum starting dilution of 8 was assigned a value of 4.
3. Correlates of protection measurements
An rSBI value ≥8 was used for correlates of protection measurements because it is a putative protective titer that predicts short-term clinical protection against disease in the United Kingdom [19]. Moreover, we used the more discriminatory rSBI value of ≥128, which reliably predicts serum bactericidal assays using human complement index ≥4 [20].
4. Statistical analysis
Descriptive statistics were used to analyze the patient characteristics, laboratory test results, and medication use. Categorical variables are presented as frequency counts, proportion percentages, and confidence intervals (CIs). We calculated the 95% CIs of the point estimates using the exact binomial distribution (Clopper-Pearson method) for proportions. For geometric mean indices (GMIs), we calculated the mean and 95% CIs on Log10 (titers/data) for a normal distribution; moreover, we applied antilog transformations. Because the GMIs were highly skewed, between-group comparisons were performed using the Mann-Whitney U test. Between-group comparisons of seropositivity prevalence were performed using the chi-square test, as appropriate. Statistical significance was set at P<0.05. Seropositive was defined by a serum bactericidal index (SBI) ≥8 or ≥128. Statistical analyses were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
5. Ethics approval and consent to participate
The study protocol was reviewed and approved by the Institutional Review Boards of Ewha Womans University Mokdong Hospital (EUMC 2015-09-043, 2020-05-022) and Seoul National University Children’s Hospital (No. 1711-106-901).
Results
1. Demographic characteristics of the participants
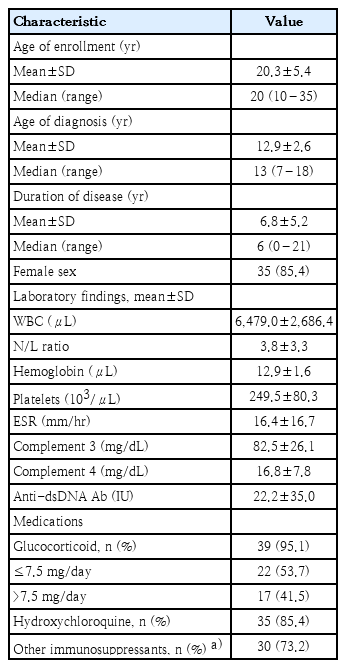
A total of 41 patients were included in this analysis. Table 1 presents the general characteristics, age at diagnosis, laboratory findings, and medications of the patients with childhood-onset SLE. The laboratory findings and medication information were based on the test results at the time of serum bactericidal activity measurement. Most patients were female (35, 85.4%), and the mean age was 20.3±5.4 years. The mean disease duration was 6.8±5.2 years. Most patients received GCs (39, 95.1%), while 85.4% and 73.2% of patients frequently used hydroxychloroquine and other immunosuppressive drugs, respectively. One patient was not receiving any immunosuppressive medication, and 3 patients were on low-dose GC or hydroxychloroquine monotherapy. The remaining 37 patients were receiving combination therapy with 2 or more medications. None of the patients had any history of meningococcal vaccination.
2. Serum bactericidal activities against meningococci
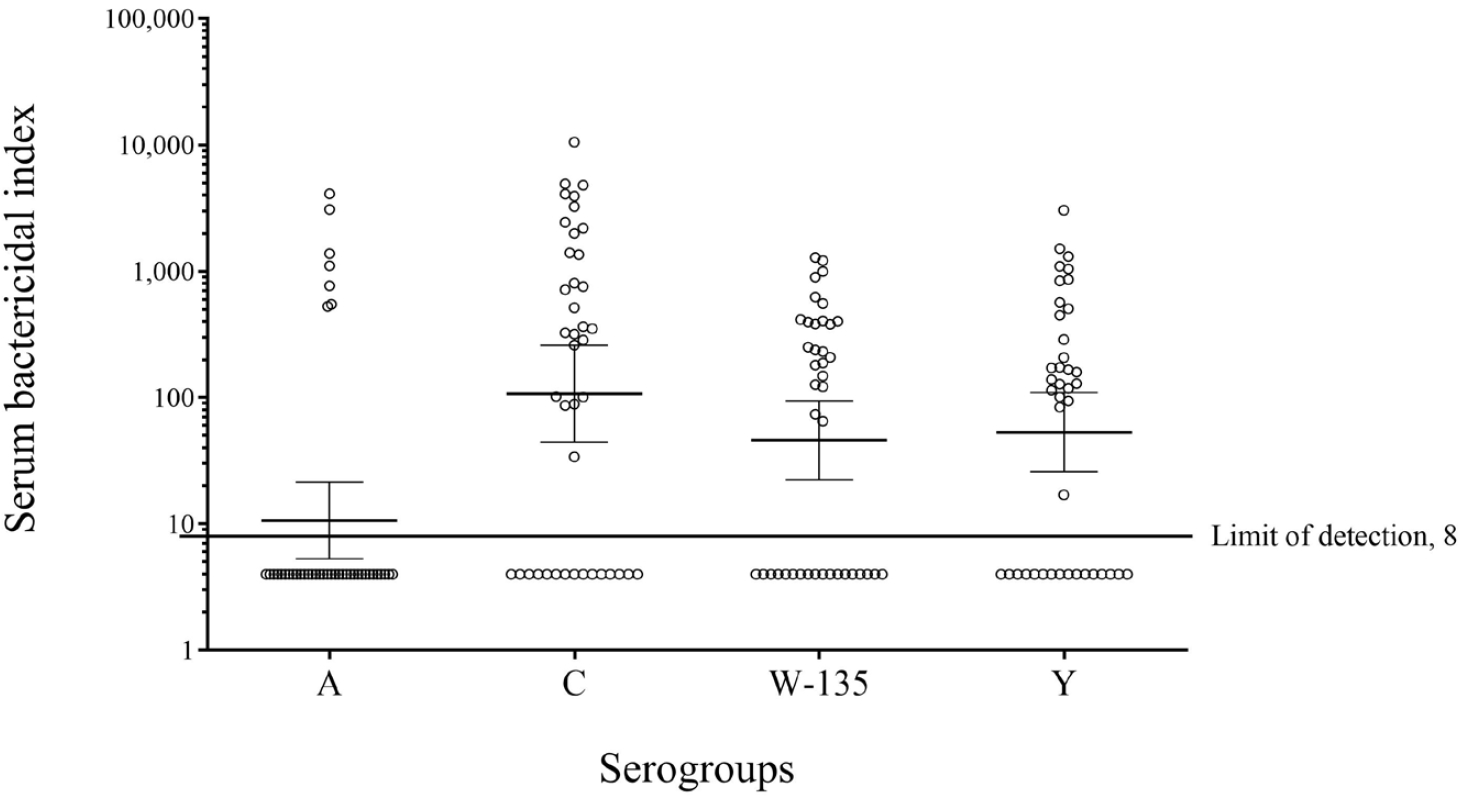
Table 2 presents the seropositivity and GMIs of the 4 meningococcal serogroups. All SBIs against the 4 meningococci in the patients are shown in a scatter plot (Fig. 1). Most patients (34 of 41, 82.9%) lacked serum bactericidal activity against serogroup A, except for 7 patients who had high SBI titers against serogroup A. Some patients showed serum bactericidal activity against serogroups C, W-135, and Y (63.4%, 56.1%, and 61.0%, respectively). There were no significant between-group differences in the seropositivity rate when SBI ≥128 was used as the cutoff for the protective level to reduce the effect of the complement source.
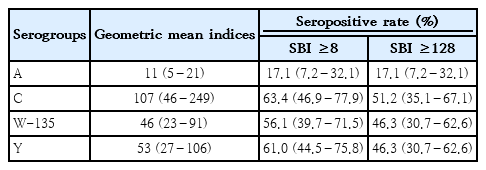
Geometric mean serum bactericidal indices (95% confidence interval) and seropositive rates by serogroup
We assessed the differences in the GMI according to the laboratory findings related to disease activity (C3 <83 mg/dL, C4 <15 mg/dL, and anti-dsDNA Ab >7 IU). In the standardized SBA method used to evaluate IgG antibodies induced by the vaccine, the influence of the patient's complement system is excluded as the assay utilizes an external complement source (from healthy individuals or rabbits), meaning the patient's complement deficiency is not reflected in the SBA. However, we analyzed the data to explore potential differences based on the patient's disease activity. For this purpose, we examined the GMIs of the subanalysis group based on laboratory findings related to disease activity, including C3 levels below 83 mg/dL, C4 levels below 15 mg/dL, and anti-dsDNA antibody levels above 7 IU (Fig. 2). No significant differences in GMIs were observed according to complement consumption state or anti-dsDNA antibody positivity. Patients who received higher GC doses (>7.5 mg/day prednisolone-equivalent) did not show significantly lower GMI titers. Finally, there was no linear correlation between the complement levels and SBIs of patients.
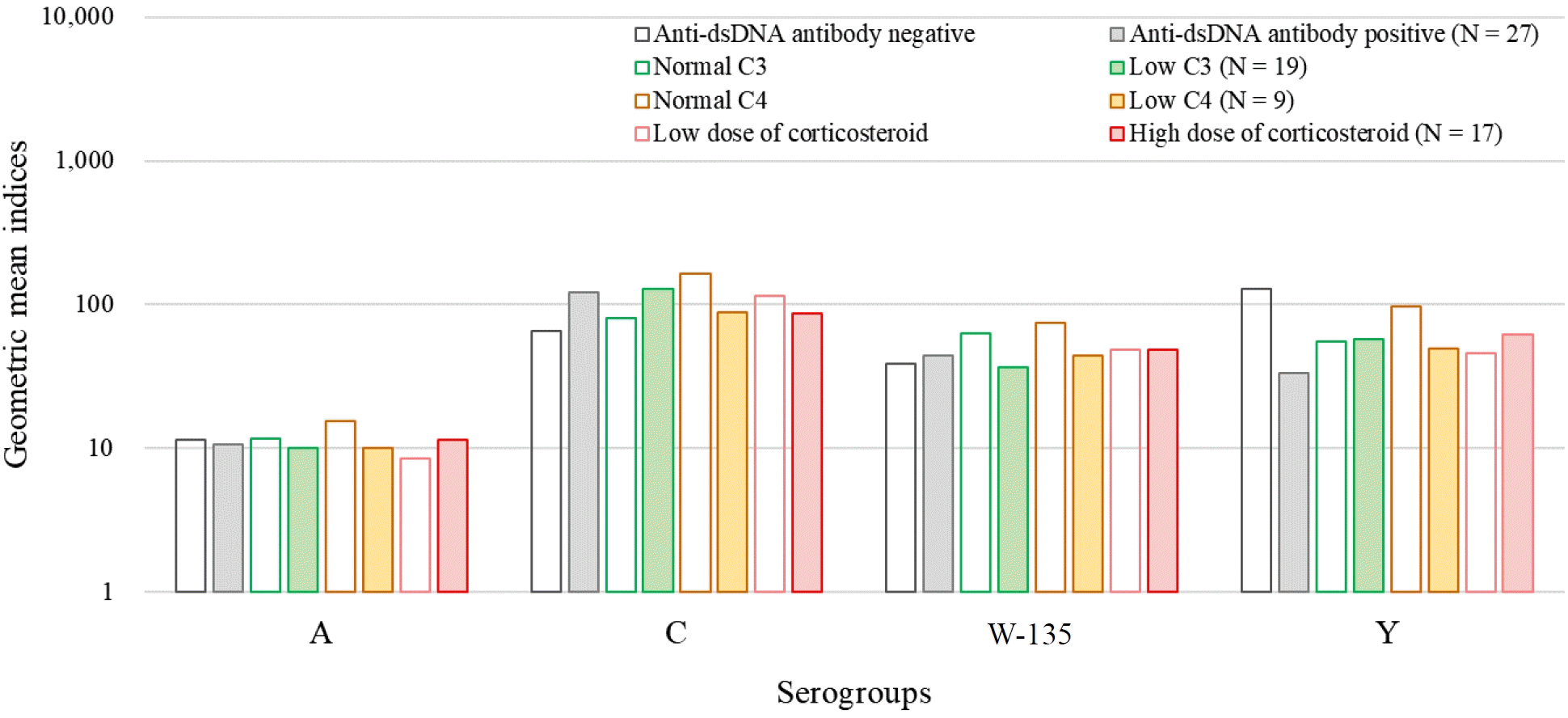
GMIs of the subanalysis based on the laboratory findings of disease activity (C3 <83 mg/dL, C4 <15 mg/dL, and anti-dsDNA Ab >7 IU). No significant differences in GMIs were noted according to complement consumption state or anti-dsDNA Ab positivity. anti-dsDNA Ab, anti-double-stranded DNA antibody; GMIs, geometric mean indices.
Discussion
We evaluated serum bactericidal activity against 4 meningococcal serogroups using sera from adolescents and young adults with SLE in Korea. Most patients were seronegative for serogroup A, whereas half of them were seronegative for the other 3 serogroups. There were no significant differences in disease activity or corticosteroid use.
The complement system is crucial in the defense against meningococcal infections [14]. Meningococcal infections are strongly associated with deficiencies in C3, properdin, and late complement components [21]. The complement profile in patients with SLE is largely similar to that of inherited complement deficiencies and defective splenic clearance of particulate immune complexes in patients with SLE [15]. Patients with active SLE have low circulating levels of complement components C3 and C4, which cause defective opsonization of immune complexes and saturation of immune complexes in the reticuloendothelial system (RES). Therefore, patients with SLE may be at risk for organisms whose removal is dependent on RES activity or complement opsonization [14]. Immunoglobulin deficiency, as well as defects in chemotaxis and phagocytic activity, may be involved in the increased susceptibility of patients with SLE to infection [14,15]. In some report, the proportion of patients with complement deficiencies diagnosed with meningococcal disease who had underlying conditions such as SLE and membranoproliferative glomerulonephritis (MPGN) was similar to those with inherited complement deficiencies. Of 20 patients presenting with their first episode of invasive meningococcal disease, 6 had low complement activity. Of these patients, 3 had acquired defects associated with either SLE or multiple myeloma [22]. Furthermore, Garty et al. [23] found that three of 30 patients with invasive meningococcal disease in Israel between 1970 and 1989 had complement deficiencies; 2 of these patients had SLE and MPGN. Reports by Feliciano et al. and Mitchell et al. further supported an association between SLE and meningococcal disease [14,21]. Despite various immunological aspects regarding susceptibility to infection in patients with SLE, cases of SLE-related Neisseria meningitidis infection remain scarce [14,15,24]. Only a few cases of SLE-related meningococcal infections have been reported; moreover, these patients often had chronic SLE and were undergoing corticosteroid treatment [21]. In this study, some patients with SLE lacked serum bactericidal activity. However, this should be interpreted through comparison with healthy individuals to determine infection susceptibility in patients with SLE.
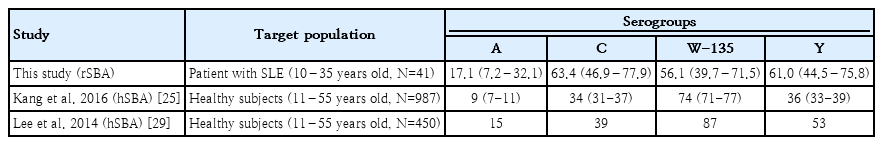
There is limited evidence regarding the epidemiology of bactericidal antibodies against meningococci in Korea. Our findings can be compared to those reported for healthy Korean people (one and 4 reports regarding seroepidemiology and immunogenicity, respectively) (Table 3) [25-29]. Although these studies used different complement sources, bacterial strains, and laboratories, these studies reported relatively high seropositivity rates in adolescents and young Korean adults. In our study, the lowest seropositivity rate was in serogroup A, which was consistent with the findings of a seroepidemiologic study conducted in a larger population (N=986) [25]. The seropositivity rates and GMIs for the other serogroups were not significantly lower than previous findings (Table 3). Although we could not perform direct comparisons, patients with SLE did not appear to have significantly lower serum bactericidal activity.
Although patients with SLE did not show significantly low serum bactericidal activity, they cannot be assumed to have a similar protective ability against meningococci as healthy individuals. Given that rSBA employs an exogenous complement (as recommended by the World Health Organization [17]) rather than complements from patients, the hypocomplement state of the patients could not affect the results. This was further indicated by the lack of differences in the SBIs according to complement levels (Fig. 2). However, there was no significant decrease in the serum bactericidal antibody levels in patients with SLE. This indicates that even with antibody production through meningococcal vaccination, the actual defense against meningococcal disease may involve other components that are not heat-labile in the plasma, such as the complement system. Although the rSBI could be a marker of vaccine immunogenicity, it cannot be used as an immunological surrogate marker for protection against meningococci. The endogenous complement of patients can be a complement source in serum bactericidal activity; however, it is difficult to collect and preserve the complement from patients, and it is impossible to standardize the assay.
Similar to other infectious diseases, vaccination is the most effective preventive measure against meningococcal diseases, and various meningococcal vaccines have been developed to prevent infections. Meningococcal vaccines are also recommended for patients with SLE due to their heightened susceptibility to infections by encapsulated bacteria, such as Neisseria meningitidis. The 2021 EULAR/PRES guidelines emphasize the importance of these vaccinations, aligning with a broader strategy to prevent infections in immunocompromised individuals. Non-live vaccines, such as meningococcal vaccines, can be safely administered to patients with SLE [30]. Seroprotection is mainly preserved in patients receiving vaccinations for immunosuppression, except for high-dose GCs and B-cell depleting therapies [30,31]. However, recommendations, including the need for repeat doses or interval periods for meningococcal vaccination, remain unclear. The available meningococcal vaccines are yet to be formally tested in patients with SLE, including functional evaluations of complement activity. Immunogenicity and efficacy data for patients with SLE are required to establish a meningococcal immunization policy. Given the low rate of recorded adverse events in vaccine recipients and the increased susceptibility of patients with SLE to infections caused by encapsulated agents, such as N. meningitidis, meningococcal vaccines remain important and effective preventive measures in these patients [32]. In Korea, unlike the vaccination policies in the United States or Europe, meningococcal vaccination is not included in the National Immunization Program due to the low incidence of meningococcal disease, which may lead to an underestimation of its necessity. However, considering the potential risk of infection, meningococcal vaccination should be considered in situations such as communal living in dormitories, travel to high-risk areas, or the use of complement inhibitors like Eculizumab.
Several studies have reported an association between disease-related markers and SLE. A higher SLE disease activity index, lower complement levels, positivity for anti-dsDNA Ab, and frequent flare-ups are significantly associated with infection in SLE [1,33-35]. Recent studies have reported that infection is correlated with treatment patterns but not with disease activity markers [8,36]. These differences could be attributed to time of collection; moreover, the treatment patterns and disease characteristics in patients were different. There are conflicting findings regarding the impact of immunosuppressive drugs on the risk of infection in patients with SLE. Some studies have reported that immunosuppressants increase the risk of infection, while others do not [1,9,10,34,35]. In our study, there was no correlation between disease activity and GC doses with GMIs.
This study had several limitations. First, we did not include matched healthy controls. Therefore, direct comparisons with healthy individuals could not be performed. Second, we included a small sample size, which may have affected the subgroup analysis. To determine the correlation between disease activity and SBIs in patients with SLE, we used complement or anti-dsDNA Ab levels as disease-related markers. However, we did not use other clinical aspects such as flare-up frequency, SLE disease activity index, or follow-up duration. Using these factors as disease activity markers in future studies could elucidate their impact on serum bactericidal activity.
Although some patients with SLE showed serum bactericidal activity against meningococci, most remained seronegative. It is important for patients with SLE who are at risk of meningococcal infection to receive appropriate vaccinations. The findings from this study could serve as serological baseline data for the meningococcal vaccination policies in SLE patients.
Notes
Conflicts of interest
HWK is a member of the editorial board of Clinical and Experimental Pediatrics. She was not involved in the editorial review or decision-making process for this manuscript. No other conflicts of interest are declared.
Funding
This study was supported by 2015 research grant to Soyoung Lee from the Korean Pediatric Society (MSD).
Author Contribution
Conceptualization: SYL, KHK, HWK; Data curation: SYL, JHL, HWK; Formal analysis: JHL, HWK; Funding acquisition: SYL; Methodology: KHK, HWK; Project administration: KHK, HWK; Visualization: HWK; Writing-orignial draft: SYL, KHK, HWK; Writing-review & editing: SYL, KHK, JHL, HWK