The role of serum zinc and selenium levels in etiology of febrile seizures
Article information
Abstract
Background
Febrile seizures (FSs) are the most common form of childhood seizures. Determining the role of trace elements in the pathophysiology of FSs will contribute to the management of FSs by pediatricians.
Purpose
This study aimed to investigate the effects of zinc and selenium on the nervous system and how they may influence the risk of FSs.
Methods
In this case-control study, there were 60 children in the simple FS group and 40 children in the complex FS group. The control groups comprised 50 children with fever but without seizures and 50 healthy children. Blood samples were collected within the first hour after FS.
Results
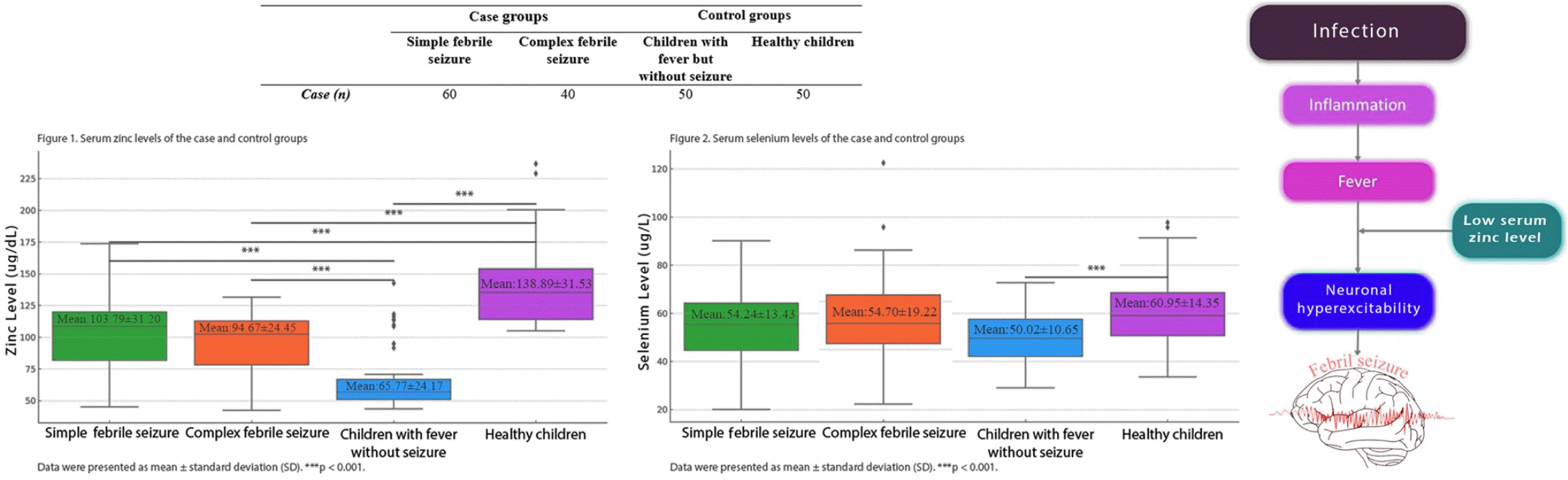
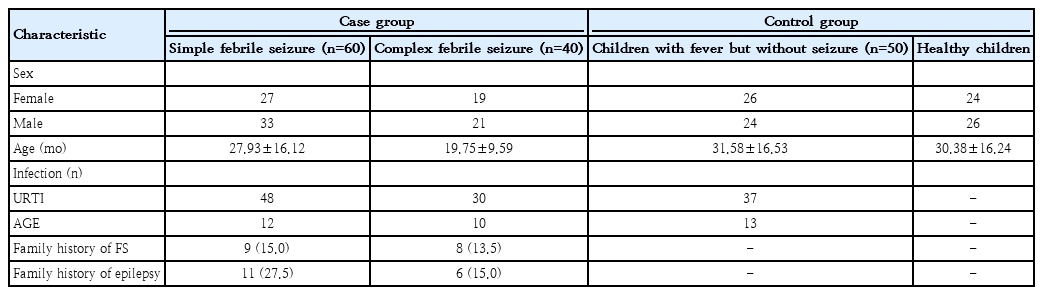
Zinc and selenium levels were significantly lower in children with fever but without seizures versus healthy children (P<0.001). Serum zinc levels were lower in children with FSs (simple and complex FSs) than in healthy children (P<0.001) but higher than in children with fever but without seizures (P<0.001). Serum selenium levels in children with FSs (simple and complex) were lower than in healthy children but higher than in the children with fever but without seizures. However, these differences were not statistically significant (P>0.05).
Conclusion
Serum zinc levels are significantly decreased during infection, whereas they show a statistically significant increase within the first hour after FS activity. This indicates that the body secretes zinc during FSs to restore homeostasis, reduce oxidative stress, and increase the seizure threshold. Therefore, zinc supplementation during febrile periods may effectively prevent FSs in high-risk children.
Key message
Zinc may play a key role in preventing febrile seizures by increasing the seizure threshold and reducing oxidative stress. Incorporating zinc supplements into treatment could help protect children from the adverse effects of febrile seizures and improve their overall outcomes.
Graphical abstract. Serum zinc and selenium levels decreased significantly during infection periods, while a significant increase in zinc levels was observed in the first hour after a febrile seizure, but no similar increase was observed in selenium. This suggests that zinc plays a role in restoring homeostasis, reducing oxidative stress, and increasing the seizure threshold.
Introduction
Febrile seizures (FSs) are defined as convulsions occurring in febrile children aged between 6 months and 5 years, in the absence of electrolyte imbalance, metabolic disorders, trauma, intoxication, a history of afebrile seizures, or central nervous system infections. FS is the most common type of seizure in childhood, occurring in approximately 2%–5% of children. FSs are categorized into simple FS and complex FS based spesific characteristics. Simple FS is defined as seizures that last for less than 15 minutes, exhibit generalized features and do not recur within 24 hours. On the other hand, complex FS involves seizures lasting more than 15 minutes, displaying focal or generalized features, and capable of recurring within 24 hours. The etiopathogenesis of FS is not fully understood. Among the predisposing factors for FS are infections, genetic factors, immunological reactions, and metabolic changes [1].
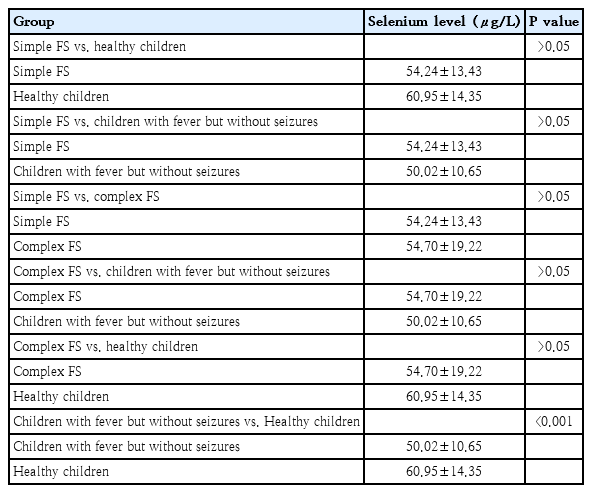
Trace elements such as zinc and selenium may play a role in the etiopathogenesis of FS due to their functions in the body [2]. Zinc is involved in enzymatic activities, protein synthesis, cellular metabolism, and nerve impulse transmission [3]. In particular, it modulates neuronal activity by influencing the pyridoxal kinase activity that regulates gamma-aminobutyric acid (GABA) levels. Additionally, it serves as a cofactor in the activation of N-methyl-D-aspartate receptors and the regulation of T-type calcium β channels [4]. Due to these properties, zinc deficiency can facilitate the occurrence of seizures. Selenium is a fundamental trace element that acts as an antioxidant, particularly in the central nervous system [5]. It is believed that selenium prevents neuronal depolarization by inhibiting free oxygen radicals through the glutathione peroxidase pathway and prevents the development of convulsions by increasing GABA levels [6].
The role of zinc and selenium in the etiopathogenesis and treatment of FS is not well understood. The results of this study will contribute to the establishment of guidelines for selenium and zinc supplementation as part of the management of FSs.
Methods
This prospective case-control study was conducted between March 2017 and December 2018 at Fırat University Hospital, a tertiary care hospital in Elazıg, Turkey. Sixty children who presented to the pediatric emergency clinic with generalized, brief seizures lasting less than 15 minutes and not recurring within 24 hours and having a body temperature above 38°C were categorized as the simple FS group. Additionally, 40 children who presented to the pediatric emergency clinic with generalized or focal seizures lasting more than 15 minutes and recurring within 24 hours and also having a body temperature above 38°C were classified as the complex FS group. Blood samples for zinc and selenium were collected from these FS patients within the first hour after experiencing the seizure.
Fifty children who presented with fever but without seizures were included as the first control group in the pediatric emergency outpatient clinic. Blood samples for zinc and selenium levels were collected from this group during the febrile period (body temperature>38°C). Fifty healthy children, who presented to the pediatric outpatient clinic for routine follow-up were included as the second control group.
Patients with chronic diseases, seizures without fever, epilepsy, history of asphyxia, evidence of central nervous system infection, structural anomalies of the central nervous system, developmental delay, electrolyte imbalances, congenital metabolic disorders or signs of systemic disease on physical examination were excluded during the creation of the case and control groups. The control group was selected from children who were similar to the case group in terms of gender, weight, and height.
The following exclusion criteria were determined for the children included in our study in terms of socioeconomic status and dietary habits that could affect zinc and selenium levels and seizure susceptibility:
1. Socioeconomic factors
Children whose socioeconomic status differed significantly (for example, according to factors such as family education level, income level or dependency on social support programs) were not included in the study. This situation was assessed using standard criteria such as the income level of the participating families and the education level of the parents.
2. Nutritional status
Children who were diagnosed with malnutrition due to inadequate or unbalanced nutrition or who reported significant weight loss or loss of appetite in the last 3 months were excluded. Additionally, children who were administered any vitamin, mineral or trace element supplements, including zinc or selenium, were also excluded from the study.
3. Chronic diseases
Children with any chronic disease that may affect zinc and selenium metabolism (e.g., malabsorption syndromes, chronic infections or inflammatory bowel diseases) were excluded from the study.
These broad exclusion criteria were created to ensure that the results of our study were based on a more reliable and homogeneous sample group.
Serum zinc level samples were analyzed using the atomic absorption spectrophotometer method on the Shımadzu AA6800 device, while serum selenium samples were analyzed using the inductively coupled plasma mass spectrometry method on the Agılent 7500 CE ICP-MS device. All parents received both written and verbal explanations of the research methodology. The children were included in the study after obtaining informed consent signed by their parents. The study was approved by the Clinical Research Ethics Committee of the Fırat University Faculty of Medicine before data collection (Decision No: 41, March 30, 2017, Fırat University). All parents were given both written and verbal explanations of the research methodology. The children were included in the study after obtaining informed consent signed by their parents.
For the statistical interpretation of the findings obtained from this study, the IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was used. The data were presented as mean±standard deviation. Differences between groups were considered statistically significant when the P value was less than 0.05. The 1-way analysis of variance (ANOVA) test was used to determine differences between groups. Post hoc Tukey test was applied to identify the source of the differences detected in the ANOVA analysis.
Results
A total of 200 children participated in our study. The case groups consisted of 60 children in the simple FS group and 40 children in the complex FS group. The control groups included 50 children with fever but without seizure children and 50 healthy children. The demographic characteristics of the study and control groups are presented in Table 1.
Serum zinc levels insimple FS and complex FS case groups were significantly lower than those in the healthy control group and significantly higher children with fever but without seizure group (P<0.001) (Table 2). Serum selenium levels of simple FS and complex FS groups were lower than the healthy control group and higher than in the children with fever but without seizure group but these differences were not statistically significant (P>0.05) (Table 3). The serum zinc and selenium levels in the children with fever but without seizure group were significantly lower than those of the healthy group (P<0.001) (Tables 2-3).
Figs. 1 and 2 present the mean serum zinc and selenium levels for the case and control groups, respectively.

Serum zinc levels by study group. Serum zinc levels in case groups (simple and complex febrile seizures) versus control groups (children with fever but without seizures, healthy). Data are presented as mean±standard deviation. ***P<0.001.

Serum selenium levels by study group. Serum selenium levels in case groups (simple and complex febrile seizure) and control groups (children with fever but without seizures, healthy). Data are presented as mean±standard deviation. ***P<0.001.
We observed FS epilepticus in 2 patients included in our study (seizure duration >30 minutes). However, due to an insufficient number of patients for statistical analysis, no additional analysis was performed.
Discussion
FSs are the most common type of seizure in childhood; however, their pathophysiology has not yet been fully elucidated [1]. There is increasing interest and discussion on the role of serum levels of trace elements, such as zinc and selenium, in the etiopathogenesis of FSs. Zinc and selenium are play a role in the etiopathogenesis of seizures due to their effects on synaptogenesis, ion channels, neurotransmitters, oxidative stress and the immune system [7-9].
During periods of infection, it has been demonstrated that plasma zinc levels rapidly decrease due to the influence of cytokines [10]. Additionally, in a swine peritonitis model, it has been reported that serum zinc levels halved within 2 hours after infection and decreased by over 75% after 6 hours [11]. Although, zinc levels tend to decrease during infections, studies have reported varying results regarding its role in FS. In the study conducted by Amiri et al. [12], the serum zinc levels of 30 patients who had FS were found to be significantly lower than the serum zinc levels of 30 children with fever but without seizures. Additionally, in studies by El-Masry et al. [2] and Arul et al. [13], the FS group also had lower zinc levels compared to the group of children with fever but without seizure. In contrast, studies conducted by Cho et al. [14] and Amouian et al. [15] did not find a significant difference in serum zinc levels between the group with FS and the group children with fever but without seizures.
In our study, serum zinc levels in both the simple and complex FS groups were found to be significantly lower than those the healthy control group. Additionally, the zinc levels in children with FS were significantly higher than those children with fever but without seizures. Furthermore, zinc levels in children with fever but without seizures were significantly lower than those healthy children.
These findings indicate that serum zinc levels decrease significantly during infection. However, it has been observed that reduced zinc levels during infection show a significant increase during seizure activity. This suggests that, during FSs, the body releases zinc to restore homeostasis, reduce oxidative stress, and increase the seizure threshold. Notably, in children, a further drop in serum zinc levels during of infection may enhance neuronal excitability, increasing the likelihood of seizure occurrence. This process highlights the potential protective role of zinc in the control of FS.
There are very few studies examining the effect of zinc supplementation on the recurrence of FSs. In the study by Shiva et al. [16], administering zinc sulfate supplementation at a dose of 1 mg/kg/day reduced the frequency of febrile illnesses but did not reduce the recurrence of FSs. Ahmada bani's study also reported that zinc sulfate supplementation at a dose of 20 mg per day did not reduce the recurrence of FSs, but this may be due to zinc deficiency secondary to infectious periods [17]. Fallah et al. [18] investigated the effect of zinc supplementation on the recurrence rate of FSs in a randomized, single-blind clinical trial comparing it with a placebo. The study included children aged 1–5 years with normal serum zinc levels who had experienced their first simple FS. Unlike other studies investigating the frequency of FSs recurrence, zinc sulfate supplementation was administered at a higher dose of 2 mg/kg/day (maximum 50 mg) per day for 6 months. The children were monitored every 3 months for 1 year for FS recurrence frequency, timing of recurrence, and side effects. The study findings revealed that zinc sulfate supplementation significantly reduced FS recurrence frequency in children with normal serum zinc levels compared to the placebo group. Moreover, the study reported that daily zinc sulfate use at a dose of 2 mg/kg/day for 6 months was safe, well-tolerated, and caused only mild and transient gastrointestinal side effects (e.g., vomiting, abdominal pain) in 16% of the children, with no severe or life-threatening events. Both the results of our study and previous studies investigating the effect of zinc sulfate supplementation on FS recurrence suggest that zinc supplementation may be beneficial in preventing FSs. For children aged 6–60 months, where FSs are most common, zinc sulfate supplementation at a dose of 2 mg/kg/day (maximum 50 mg) during infection periods or seasons when infections are frequent could reduce seizure risk. Particularly, children with genetic mutations predisposing them to FSs, malnutrition or micronutrient deficiencies, significantly reduced serum zinc levels during infections, a family history of FSs, seizures at low-grade fever, or recurrent FSs may be identified as the target population for zinc supplementation. Since studies investigating the effect of zinc supplementation on FS recurrence are both very limited in number and concentrated in certain geographic regions, multicenter randomized controlled trials are needed to determine the timing and dose of zinc supplementation in this target population with high-risk factors for FSs.
Low serum selenium levels facilitate the formation of seizures by increasing glutamate-related excitotoxicity and disrupting neurotransmitter functions due to increased oxidative damage [8]. In many studies, it has been reported that serum selenium levels decrease with the increase of inflammatory biomarkers during infection periods [19,20]. The role of serum selenium levels in FSs is not well understood, as studies on this topic are limited in number and generally involve small patient samples. Abuhandan et al. [21] reported that children with FSs had lower selenium levels than children with fever but without seizure. In another study, no statistically significant difference was found serum selenium levels between children with FSs and children with fever but without seizure [22].
In our study, selenium levels in both the simple FS and complex FS groups were lower than those in the healthy control group however higher than in the children with fever but without seizure group. However, these differences were not statistically significant. Additionally, selenium levels in children with fever but without seizure were found to be significantly lower those in than healthy children.
Our study results indicate a significant decrease in serum selenium levels during of infection but show no significant increase in serum selenium levels during seizure activity. During FS, the body may utilize alternative antioxidant mechanisms, such as vitamin E, vitamin C, or glutathione, to maintain oxidative balance. This may explain why hischanges in serum selenium levels do not show a significant association with seizures. Additionally, genetic variations can affect selenium metabolism, making some individuals more sensitive to this micronutrient. The lack of statistical significance in differences between selenium levels may be due to the limitations of the available sample size in the study groups. To better understand this situation, we performed a power analysis simulation including all group combinations (Fig. 3). Our power analysis results indicate that the possibility of detecting smaller differences in selenium levels with our available sample size is limited. In this context, we think that the lack of statistical significance in our selenium results may be due to the lack of sufficient statistical power and sample size. Our findings suggest that selenium does not play a critical role in the pathophysiology of FSs. However, although no significant changes in serum selenium levels have been observed during FSs, there may still be alterations in selenium levels within brain tissue or specific selenoproteins. Therefore, cellular-level analyses examining the effects of selenium could provide a clearer understanding of its relationship with seizures. In addition, there is a need for future multicenter studies with larger sample groups and sufficient statistical power.

Power analysis of selenium level comparison. Results of the power analysis for selenium level comparisons across all group pairs are shown. The graph demonstrates how statistical power improves as sample sizes increase, highlighting the limitations of the current sample size for detecting small intergroup differences.
This study is one of the first comprehensive investigations conducted with 2 case groups, consisting of subgroups of FSs (simple FS and complex FS) and 2 control groups, consisting of children with fever but without seizures and healthy children. Additionally, unlike previous studies, serum samples were collected within the first hour after FSs. These results allow for a clearer evaluation of biochemical changes in serum zinc and selenium levels during both febrile periods and FS. The fact that serum zinc and selenium levels tended to increase during FSs activity will make an important contribution to the literature.
An important limitation of our study is the inability to measure preseizure serum levels of zinc and selenium in patients with FSs. The challenges associated with predicting which patients will develop seizures during febrile episodes further elucidate this limitation. FSs are multifactorial, with genetic predisposition potentially playing a significant role in their pathogenesis. Specific genetic variants may influence the metabolism, bioavailability, and cellular functions of trace elements, such as zinc and selenium, thereby increasing susceptibility to seizure development. Consequently, genetic variants, in conjunction with the neurophysiological effects of these trace elements, are critical factors in the manifestation of seizures.
In light of these considerations, future research endeavors should focus on comparing preseizure and postseizure serum levels of zinc and selenium, while accounting for genetic variations. Such investigations will enhance our understanding of the relationship between FSs and these trace elements and may contribute to the development of potential therapeutic strategies.
In conclusion, we suggest that decreased serum zinc levels during periods of infection may play a role in the pathogenesis of FSs. We suggest that zinc supplementation may be beneficial during febrile periods, especially in children with certain genetic variants associated with FSs, a family history of FSs, and high-risk factors such as a history of recurrent FSs.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
We would like to thank all the children and families who participated in the study. We would like to thank the hospital staff who provided support at every stage of this study for their important contributions.
Author Contribution
Conceptualization: YA; Data curation: YA; Formal analysis: HGP; Methodology: YA; Project administration: YA; Writing - original draft: YA; Writing - review & editing: YA, HGP