Incidence of neural tube defects in tertiary care university hospital in Bangladesh
Article information
Abstract
Background
Although national population-based birth defect prevalence estimates are unavailable for Bangladesh specifically, data extrapolated from the March Dimes Global Birth Defects Report indicate a prevalence of neural tube defects (NTDs) of 4.7 per 1,000 live births.
Purpose
This study aimed to determine the prevalence of NTD among infants born at a tertiary care multidisciplinary referral hospital in Bangladesh.
Methods
Live born infants with NTD were prospectively enrolled in 2015–2021. Each enrolled NTD case was examined for type, location, and associated anomalies. The overall and annual prevalence rates were then calculated.
Results
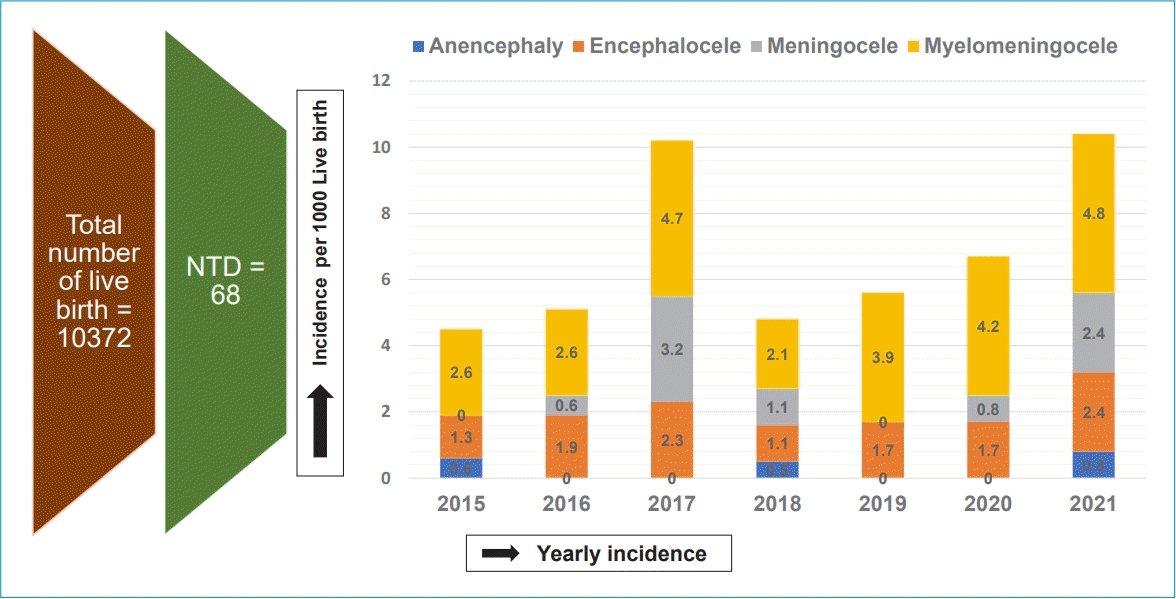
A total of 10,372 newborns were enrolled; of them, 68 had NTD (incidence, 6.4 [range, 4.59–11.2] per 1,000 live births). The mean maternal age was 27.49± 4.72 years. Three-quarters of the NTD cases were detected at birth, and 94% of the mothers reported not taking periconceptional folic acid supplements. The meningomyelocele complex was the most frequent location. Two peaks in incidence were noted in 2017 and 2021 (10.28 and 11.2 per 1,000 live births, respectively). The distribution of different NTD types included meningomyelocele at 53%, encephalocele at 26.6%, meningocele at 16%, and anencephaly at 4.4%. A male predominance was noted overall except for anencephaly. The most common location was the lumbosacrum (47%). The NTD was isolated in 20.59% (14 of 68) of cases and associated with other malformations in 80% (54 of 68) of cases.
Conclusion
The incidence of NTD was 6.4 per 1,000 live births at a leading tertiary care multidisciplinary referral center in Bangladesh. However, this figure might not reflect the incidence of NTD in the wider population.
Key message
Question: What is the burden of neural tube defects (NTDs) in a tertiary care neonatal intensive care unit in Bangladesh?
Finding: The overall incidence of NTD was 6.4 (range, 4.59–11.2) per 1,000 live births, and the meningomyelocele complex was the most frequent location.
Meaning: The high incidence of NTD found in a leading tertiary care multidisciplinary referral hospital in Bangladesh may not reflect that of the wider population.
Graphical abstract. Yearly incidence of different types of neural tube defect (NTD).
Introduction
Neural tube defects (NTDs) are serious congenital anomalies [1], caused by partial or incomplete closure of the neural tube during embryogenesis [2]. Globally in 2015, an estimated 260,100 NTD-affected birth outcome with prevalence of 18.6 (15.3–23.0)/10,000 live birth was reported and nearly 75% (117,900) resulted in under -5 deaths among NTD-affected live births [3]. Overall NTD burden on live births expressed in median from 18 countries in 6 World Health Organization (WHO) region was 1.67/1,000 (interquartile range, 0.98–3.49) [4]. In low and middle income countries, 29% of neonatal deaths are attributed by NTDs [5]. Although the national population-based birth defects prevalence estimates are not available in Bangladesh according to the March of Dimes Global Report on Birth Defects the extrapolated data indicated the NTD prevalence as 4.7 per 1,000 live births [6]. Estimate from hospital based studies is between 10.4 and 38.2 per 10,000 births which is higher than the global NTD prevalence [7-9].
The etiology of NTDs is multifactorial and can be attributed to genetic predisposition, vitamin deficiencies, folate deficiencies or exposure to teratogen [10]. Lack of awareness regarding beneficial effect of folate supplementation coupled with high rate of unplanned pregnancy has led to underutilization of preventive strategies especially in resource limited settings [11]. Despite WHO recommendations for the use of folic acid supplements among women of childbearing age [12], prevalence of prenatal folic acid supplement intake is low (~20%) in Bangladesh [13]. Bangladesh also has a high prevalence of malnutrition and micronutrient deficiencies hindering optimal birth outcomes [14]. According to the Food Fortification Initiative Bangladesh does not currently have a national program for mandatory folic acid fortification [15]. Moreover, investigators’ ascertain metalloid especially arsenic exposure as a potential risk factor for the development of NTD as it can modify the effect of folic acid supplementation in Bangladesh [16,17].
Given the country’s exposure to folate and other micronutrient deficiency [18] it is essentially important to report NTD incidence to get the insight of actual burden of Bangladesh. Objective of this prospective study was to report prevalence of NTD and to describe antenatal and perinatal characteristics, distribution and systemic association, in the Department of Neonatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Methods
This prospective descriptive study was conducted over a period of 7 years from January 2015 to December 2021 in the neonatal intensive care unit (NICU) of a tertiary care hospital, BSMMU, Dhaka, Bangladesh. All liveborn inborn neonate delivered with the diagnosis of NTD were enrolled and examined after taking informed written consent. Stillborn with features of NTD and outborn cases were excluded from the study Department of neonatology, BSMMU is a 31-bedded tertiary care newborn unit which serves both inborn and outborn referred cases. The neonatology department collaborates with pediatric surgery department for surgical intervention of NTD patients. The university have countries leading feto-maternal unit in the Obstetrics and Gynecology department dedicated to provide care for high risk pregnancies, referred from different parts of the country
During the study period, the unit was acting as nodal center for Bangladesh National Neonatal Perinatal database (NNPD) which was established in 2013 as a continuation of the WHO-Regional office for South-East Asia database. The birth defect surveillance program was intended to commence NNPD in the referral hospitals of Bangladesh with the objective to set up a network for collection of neonatal perinatal data for better understanding of epidemiology of neonatal diseases including birth defects. Later on considering the importance of surveillance of birth defect, it was strengthened as “Newborn Birth Defect (NBBD) surveillance in Bangladesh.” This program was going on in 20 medical colleges and institutes in Bangladesh and supported by WHO and Directorate General of Health Services, Ministry of health and family welfare, Bangladesh for better understanding of epidemiology and to develop strategic framework for the care and prevention of birth defect in this region (https://apps.who.int/iris/handle/10665/204821) [19].
Maternal variables including age, parity, consanguinity, maternal pregestational and/or gestational diabetes, hypertension, information on periconceptional folic acid supplementation, previous sib affected with NTD and positive family history of NTD were collected from the mother and obstetrician. Gestational age of detection of NTD by antenatal ultrasonogram scan was also recorded. Perinatal characteristics included birth weight, gender distribution, gestational age, mode of delivery and place of delivery were collected in the data collection form by the attending residents. Each enrolled newborn was examined by the consultants for accurate birth defects description. Each data collection form was also checked by the lead investigator of the study.
Detail descriptions of NTD types, localization, isolated or associated with other malformation were recorded in the data collection form after reviewing the clinical description of the individual NTD forms, attached photographs and available investigation reports. Postnatal age of surgical intervention, name of the surgical procedure, outcome of surgery was collected. Overall outcome in terms of discharge from NICU, left against medical advice and death were documented. Incidence of NTD was calculated by dividing the number of live born NTD by the total number of live born during the study period (2015-2021), and multiplying by 1,000 to obtain the prevalence of NTD per 1,000 live born neonate.
NTDs identification and correct description was reported using the standard operating procedure of the database and atlas of selected congenital anomalies. Following definitions were used [20].
1. Liveborn NTD
An alive newborn with any type of visible NTDs at birth (anencephaly, spina bifida, or encephalocele, or myelomeningocele or meningocele), irrespective of gestational age.
2. Neural tube defect
Liveborn baby with single or multiple defects including craniorachischisis, anencephaly, myelocele-myelomeningocele, meningocele, encephalocele/cephalocele.
3. Anencephaly
Anencephaly is an anterior neuropore closure anomaly with the neural tube in a state of neural tube, with an extracranial protrusion of the brain. The latter will suffer from aggression and decay due to the contact with amniotic fluid to cause anencephaly.
4. Spina bifida
Spinal cord closure defect characterized by open posterior arches of the thoracic, lumbar or sacral spine and visible herniation of meninges or meninges-spinal cord.
5. Myelocele/myelomeningocele
Spinal cord closure defect characterized by open posterior arches of the thoracic, lumbar or sacral spine and visible herniation of the meninges and spinal cord.
6. Meningocele
Spinal cord closure defect characterized by open posterior arches of the thoracic, lumbar or sacral spine and visible herniation of the meninges only.
7. Encephalocele
Cystic lesion through a defect in the skull, pedunculated or sessile.
Ethical approval (registration number 653) was taken from Institutional Review Board of BSMMU. The statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co.). Quantitative variables are expressed in mean±standard deviation and qualitative variables in numbers and percentages.
Results
During the study period from January 2015 to December 2021, 10372 births were enrolled including 68 cases of inborn NTDs give rise to an overall incidence of 6.4 per 1000 live births.
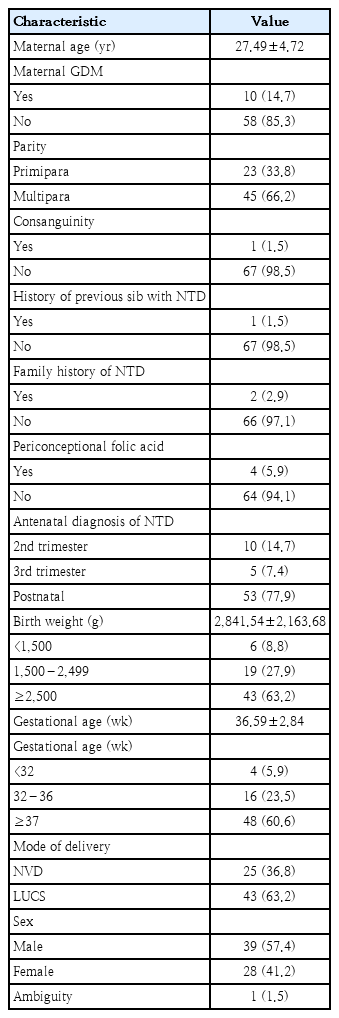
Baseline perinatal characteristics are shown in Table 1. Maternal age mean±standard deviation was 27.49±4.72 years. Consanguinity was documented in 1.5% and maternal diabetes was recorded in 14% cases. Previous sibling with NTD and similar defect in the family was reported in 1.5% and 2.9% cases. Three quarter NTD cases were detected at birth and 94% did not take periconceptional folic acid supplementation. Mean birth weight and gestational age was 2841.54±2163.68 g and 36.59±2.84 weeks respectively. Data showed overall male predominance 57% vs 41% and ambiguity 1.5%. Sixty-three percent NTD cases were delivered by lower uterine cesarean section.
Fig. 1 shows year wise trends of incidence rate of total number of NTD including subtypes during the study duration. Reported incidence ranges from 4.59–11.2 per thousand live birth. Two peaks were noted in the year 2017 and 2021 with an estimated incidence rate of 10.28 and 11.2 per thousand live births respectively. Meningomyelocele complex was most frequent NTD types throughout the study years. Two peaks in 2017 and 2021 were noted in myelomeningocele complex with incidences 4.7 and 4.2 per thousand live births respectively. Similar Meningocele peaks were noted in the year 2017 and 2021 with an incidence rate of 3.2 and 2.4 per thousand live births. Encephalocele incidence rates were similar during the study period (1.1 to 2.4 per thousand live births). Anencephaly detection rate is low (0.5 to 0.8 per thousand live births) throughout the study period.
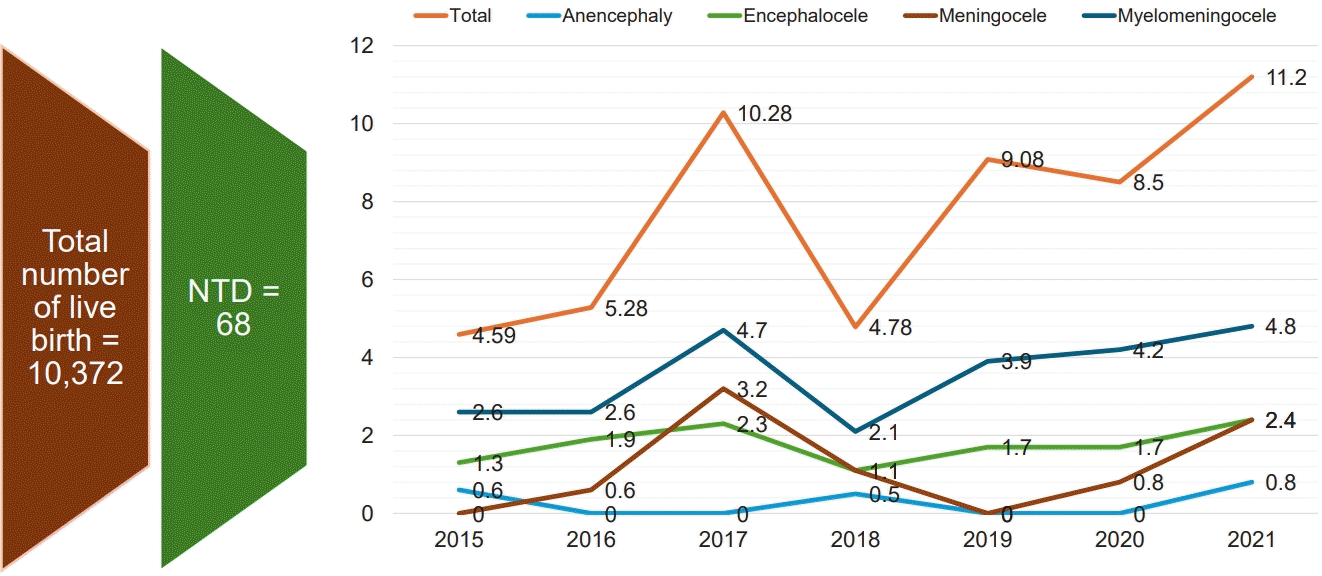
Yearly incidence of neural tube defects per 1,000 live births overall and by subtype. NTD, neural tube defect.
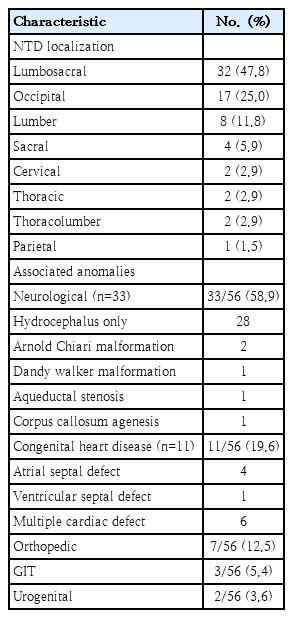
Distribution of different NTD subtypes included meningomyelocele 53%, encephalocele 26.6%, meningocele 16% and anencephaly 4.4%. Overall, male predominance was noted except anencephaly. The most common location was lumbosacral region (47.0%), next common locations were occipital (25.0%), lumber (12.0%) and sacral region (5.9%). The NTDs were isolated in 14 of 68, 20.59% of cases and associated with other malformations in 56 of 68, 82.3% of cases. Among NTD cases associated with other congenital anomalies, neurologic defects were associated in 58.9% (33 of 56) of cases; hydrocephalus being the most frequent association 28 of 33 (85%). Cardiac and orthopedic anomaly was associated in 19.6% (11 of 56) and 12.5% (7 of 56) of cases respectively. Among cardiovascular defects, 36% (4 of 11) was atrial septal defect and 54% (6 of 11) multiple cardiac defects (Table 2).
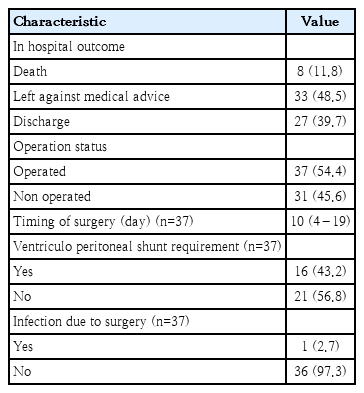
Table 3 demonstrated outcome of the NTD cases. Nearly 40% patients were discharged while 48% left against medical advice. Deaths during the hospital stay were attributable in 12% cases. NTD patients who underwent surgical intervention (n=37), median age at operation were 10 days.
Discussion
In this prospective study, 68 inborn cases of NTD were reported, thus the prevalence rate is 6.4 per 1,000 births. This figure is high compared to the global incidence (1.8/1,000 live birth) [6]. The current study’s NTD incidence also outnumbered the previous NTD reports in Bangladesh based on public hospitals [7-9], and March of Dimes Global Report on Birth Defects [6]. In an Indian Study conducted in Northern part, reported incidence is higher than our study; 7.48 per 1,000 live births [21].
Several general factors namely type of study setting (referring vs referral center and primary vs. tertiary level multidisciplinary service center), enrolled population (population-based vs. hospital based) and geographic location may be associated with the high NTD incidence reported in the current study. Apart from these factors, other important explanations behind the higher NTD incidence in Bangladesh than other countries can be summarized as follows. First of all, exposure to many risk factors such as dietary micronutrients and folate deficiency [14], arsenic metalloid exposure [16,17], and few other reasons e.g., consanguineous marriage [22], maternal diabetes mellitus, teratogen exposure might be the reason for a higher incidence of NTDs compared to that of the high-income countries and other Asian countries. As this study intended to report the burden of the NTD hence analysis between the potential factors with the development of NTD is lacking. Secondly, high rates of unplanned pregnancy [23], delay in first antenatal care seeking by the pregnant women, suboptimum antenatal care in terms of compliance of care components [24], might lead to delay in prenatal diagnosis of NTD in resource limited setting like Bangladesh. This in turn, might be associated with unwillingness for termination of affected pregnancy in later gestation. Reluctance to medical termination on religious point of view may be another important reason behind the high incidence of live birth NTD. Thirdly, the current study setting is the leading tertiary care referral university hospital with only fetomaternal department in the country during the study period which caters referred high risk complicated pregnancies including NTD-affected cases. Comparative representation of Bangladesh NTD rates along with other Asian countries (India, China, Japan and South Korea) [25-28], after 2005 is shown in Table 4. Moreover, it is well known that the South-East Asian region is lack in surveillance and registry data despite being exposed to large portion of the global population with high disease burden. Thus, methodological variation, variation in population inclusion criteria and coverage gap in NTD data collection can play an important role in wide range in reported incidence.
The yearly incidence noticed more than doubled (4.59 in 2015 vs. 11.2 in 2021) during the study period that reflect increased referral by the network participating hospital under the surveillance program. Although the high incidence rate reported in our study is unlikely to be representative of the general population, the estimated burden from surveillance database will provide a useful initial assessment of the NTD burden in Bangladesh.
In this study, trends of yearly incidence of overall NTD range from 4.59–11.2 per thousand live birth. Two peaks were noted in the year 2017 and 2021 with an estimated incidence rate of 10.28 and 11.2 per thousand live births respectively. Trend of yearly incidence rate of different NTD type shows meningomyelocele complex was most frequent NTD types throughout the study years. Two peaks in 2017 and 2021 were noted in myelomeningocele complex with incidences 4.7 and 4.2 per thousand live births respectively. Although the exact reason behind the 2 peaks were not analyzed as part of the report, incidental increase in referral might explain the scenario. Similar Meningocele peaks were noted in the year 2017 and 2021 with an incidence rate of 3.2 and 2.4 per thousand live births. Encephalocele incidence rates were similar during the study period (1.1 to 2.4 per thousand live births). Anencephaly detection rate is low (0.5 to 0.8 per thousand live births) throughout the study period. Similar trend of yearly incidence of NTD and different types of NTD has also been reported by a recent Moroccan study by Forci et al. [29] which shows low incidence rate of overall NTD and types throughout the study period of 2011 to 2016.
Studies has been demonstrated the role of consanguinity as a risk factor for developing NTDs [30], although a recent systematic review did not find significant relation between consanguineous marriage and NTD [31]. The consanguinity rate is low (1.5%) in our study. Among women who have had a NTD-affected pregnancy, recurrence risk is markedly higher than the risk for a first NTD-affected pregnancy in the general population [32]. In our NTD series, family history of similar NTD was found only in 3.12% of cases.
Folic acid supplementation reduces NTD recurrence risk by 40 to 80% and it is effective when taken during periconceptional period and throughout the first trimester [33]. However, only 6.9% mothers of the current study receive periconceptional folic acid. Finding is similar with 2 relevant studies conducted in Turkey and Morocco [29,34]. This finding recommends that the necessity of mass awareness regarding the efficacy of folic acid intake during periconceptional period and country adoption of food fortification of folic acid.
Maternal diabetes mellitus has an important association with NTD. NTD was reported to increase by 4.2 times among diabetic women in a study done in United Kingdom by Macintosh et al. [35]. In our study, 14.7% mothers had type 1 diabetes mellitus.
Antenatal ultrasonography is useful for the identification, type and location of the NTD. Thus, it allows discussion with parents regarding therapeutic termination of pregnancy. Characteristic 2-dimensional antenatal sonographic signs (lemon shaped head, banana cerebellum, ventriculomegaly) are important diagnostic clues to the presence of spina bifida. However, only 14% of our NTD cases were diagnosed during 2nd trimester which could allow parents to be briefed on the possible option of therapeutic termination. Nearly 80% of our NTD cases were detected after delivery. Contrasting to our findings, about two-thirds (63%) of NTD cases were diagnosed antenatally with only 4 cases (9.1%) who underwent a pregnancy termination procedure in a recent Moroccan study [29].
Meningomyelocele (46%) was the most common type of NTD reported in our study. Next to meningomyelocele, encephalocele (26.5%) and meningocele (15%) were the frequent types. In this NTD series, anencephaly was found in 4.4% cases. These findings are similar with that of a Turkish study done in 2018 [34]. The higher frequency of anencephaly in this earlier study may be explained by unaware/ unknown antenatal detection which have been improving in the recent years in many low and middle income countries including Bangladesh.
The most common location was lumbosacral region (47%), next common locations were occipital (25%), lumber (12%) and sacral region (6%) (Table 2). The most common site of NTD defect is in the lumbar region according to the available literature. The present study findings were similar with the report. Turkish study observed that 39.2% defect was in the lumbar region, 24.6% in the lumbosacral region and 18.6% in the thoracolumbar region [34].
The most common neurologic association with NTD is hydrocephalus; rate is 80%–96% in open spina bifida cases. In our NTD series, the association of hydrocephalus was documented as more than (28 of 33) 85% among those having neurologic association. This finding is in line with the available literature. The second most common cranial anomaly is Chiari malformation. The detection of neurologic association among NTD cases is difficult in the present study setting as the neuro imaging evaluation need to ensure clinical stability to transfer the baby to Magnetic Resonance Imaging unit. Parental affordability and cost of detail neuroimaging is also a concern.
Neural crest cells play a critical role in the development of heart, urinary system, and skeleton. Therefore, cardiovascular defect, renal and skeletal abnormality may be associated with NTDs. In our study, we found that 20% of NTD patients had cardiac defects. Four NTD patients had atrial septal defect and 6 had multiple congenital heart defects. Median age of surgical intervention in our NTD cases was 10 days. The delay in surgical intervention was mostly due to pediatric surgery patient load in the study settings.
The mortality rate was 12% in our study. Nearly half of the parents of NTD left against medical advice in view of guarded prognosis even after surgical correction. Left against medical advice anticipating unfavorable outcome might lead to possible underestimation of actual mortality rate in the current study.
This is the first attempt to report incidence trend of NTD from a tertiary care referral university hospital over a period of 7 years in Bangladesh.
One of the important limitation of this study is that this study provides single center tertiary referral university hospital finding which certainly not representative of the country population. As this study was intended to document the incidence, the study lack the exploration of association between potential country context risk factors with the development of NTD. Other important characteristics especially socieodemographic and some maternal factors (pyrexia, exposure to drug or teratogen etc.) were not encountered in the study. There is an urgent need to conduct large multicenter involving public hospitals and community to get the national level NTD incidence and possible risk factors to develop implementation of primary prevention program and to prove the potential benefit of folic acid supplementation and fortification.
The overall incidence of NTD is 6.4 per 1,000 live births in this study. Incidence trend ranges from 4.59– 11.2 per thousand live birth and meningomyelocele complex was the most frequent throughout the study duration. The reported high incidence is representation of a leading tertiary care multidisciplinary referral center in Bangladesh which might not be the reflection of the population. Thus, careful interpretation is needed before generalizing the study findings.
This study reminds the necessity of nationwide surveillance of the actual burden of the NTD to formulate primary preventive strategies including mass awareness on birth defect, food fortification with folic acid and folic acid supplementation for women of reproductive age. Researchers should investigate the association of country context potential risk factors for the development of higher incidence of NTD in comparison to the other region of the world. Government and non- government organization should focus more on trimester specific regular antenatal care coverage for early detection of pregnancy complicated with NTDs so that medical termination can be offered to the parents in view of possible adverse outcome. NTD identified at birth should be offered with more organized, multidisciplinary care in an advance center having facility for long term follow up. Genetic counseling and possibility of NTD on the subsequent pregnancy should be informed to the affected family with emphasis of the preventive measures.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Conceptualization: IJ; Data collection: AH, SNS; Investigation: PW, PN; Methodology: SCM, KHS; Project Administration: MS; Writing – Original Draft: IJ; Writing – Review & Editing: MS, SKD, MAM
Funding
WHO-Regional office for South-East Asia funded the activities of Newborn Birth Defect project in the participating centers including Bangabandhu Sheikh Mujib Medical University.
Acknowledgments
We express sincere gratitude to all parents for participation. We appreciate the residents, staff nurses of NICU for NTD case ascertainment, capturing the photograph and caring for the baby. We also feel grateful to the pediatric surgery department for surgical management of the baby.