Evaluation of Bak and Bcl-Xl gene expression among pediatric patients with acute primary immune thrombocytopenia
Article information
Abstract
Background
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by a low platelet counts and an increased risk of bleeding. Moreover, the apoptotic mechanisms of platelets may influence their production and lifespan.
Purpose
To assess the involvement of apoptotic markers—specifically the B-cell lymphoma protein 2 family proteins Bak and Bcl-Xl in the pathogenesis of acute primary ITP in pediatric patients, and to evaluate the impact of intravenous immunoglobulin (IVIG) therapy on their expression.
Methods
This study included 24 children with acute primary ITP and 30 healthy controls. Patients were enrolled from the Hematology and Oncology Unit of Menoufia University Hospitals, Egypt. Two peripheral blood samples were obtained from each participant: one prior to receiving IVIG therapy and the other after treatment. Platelet-rich plasma was isolated, and Bak and Bcl-Xl gene expression levels were assessed using reverse transcription quantitative polymerase chain reaction.
Results
Before treatment, Bak gene expression and Bak/Bcl-Xl expression ratio were significantly higher in patients versus controls (P=0.001 and P<0.001, respectively), whereas Bcl-Xl gene expression was significantly lower (P=0.029). After treatment, Bak gene expression and the Bak/Bcl-Xl expression ratio decreased significantly (P<0.001 and P=0.001, respectively), whereas Bcl-Xl gene expression increased significantly (P<0.001).
Conclusion
Pediatric patients with acute primary ITP exhibited a heightened proapoptotic state, as indicated by an increased Bak expression and Bak/Bcl-Xl expression ratio, as well as a reduced Bcl-Xl expression. IVIG therapy appears to mitigate this pro-apoptotic effect, suggesting its ability to restore platelet homeostasis.
Key message
The B-cell lymphoma protein 2 family proteins Bak and Bcl- Xl, important markers of apoptosis, may contribute to primary immune thrombocytopenia (ITP). Thus, their expression may serve as biomarkers for the diagnosis and monitoring of pediatric ITP. Targeting these pathways may improve platelet survival, particularly in treatment-resistant cases. Personalized treatments based on apoptotic profiles can optimize therapy and reduce the unnecessary use of immunosuppressive drugs.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmunity hematologic disorder marked by a reduced platelet count and considerable clinical variability [1]. It is primarily driven by an immune response in which immunoglobulin G (IgG) autoantibodies directed against platelet surface glycoproteins (most notably GPIIb/IIIa and GPIb/IX) leading to increased platelet destruction [2].
ITP most commonly presents with symptoms of bleeding, which can present as purpura, epistaxis, menorrhagia, gingival bleeding, or gastrointestinal (GIT) hemorrhage [3]. In pediatric populations, the annual incidence of ITP is estimated at around 12 cases per 100,000 children, with severe cases carrying a yearly mortality risk of 1%–3% [2,4]. ITP is classified as either primary or secondary. In primary thrombocytopenia (idiopathic), no clear initiating or underlying cause is identified. In contrast, secondary ITP is typically related to a causative condition or trigger, like medications, vaccinations, infections, and other factors [5-7].
The International Working Group classifies ITP into 3 categories based on the duration of low platelet counts: newly diagnosed ITP (within the first 3 months), persistent ITP (lasting from 3 months to 1 year), and chronic ITP (lasting longer than one year) [4].
Apoptosis, a programmed process of cellular death, is important in maintaining cell turnover and removing damaged or infected nucleated cells [8]. It primarily involves 2 pathways: the death receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway [9]. Numerous studies have demonstrated that platelets possess the molecular machinery and internal pathways required for apoptosis, which in turn influence their viability and life span [10,11].
B-cell lymphoma protein 2 is a group of proteins that control the intrinsic apoptotic pathway. This protein family comprises both proapoptotic factors (like BAD and Bak) and antiapoptotic factors, including Bcl-Xl. Intrinsic apoptotic pathway is triggered by alterations of mitochondrial outer membrane, consequently, cytochrome c is liberated into the cytosol and subsequently forms the apoptosome complex by binding the apoptotic protease-activating factor 1 and procaspase-9, that in turn stimulates caspase-9, initiating a cascade that leads to the stimulation of downstream effector caspases [12].
The apoptotic pathway (extrinsic) is initiated when 'death receptors' (tumor necrosis factor receptor superfamily members possessing intracellular death domains) form complexes with their designated ligands. This interaction triggers the recruitment and activation of the initiator enzyme procaspase-8, which subsequently stimulates the downstream activation of effector caspases, including caspase-3, caspase-6, and caspase-7 [12].
Recent research has highlighted that apoptosis occurs within platelets and controls both their production and lifespan. Moreover, antiplatelet antibodies, known to induce thrombocytopenia in murine models, have been correlated with increased apoptotic markers in platelets [13].
This study aimed to explore the involvement of Bak and Bcl-Xl proteins in the evolution and progression of acute primary ITP in children, compared to a group of healthy controls. In addition, it sought to evaluate how intravenous immunoglobulin (IVIG) therapy affects the expression of these apoptotic markers in pediatric ITP patients.
Methods
This cross-sectional, case-control study included 24 pediatric patients with acute primary ITP (11 boys and 13 girls) and 30 healthy controls (14 boys, 16 girls). Although sample size calculations for detecting a large effect size with 80% power at a 5% significance level typically recommend approximately 26 participants per group, the number of patients in this study was limited to 24 due to the availability of eligible cases during the study period. However, the control group comprised 30 participants, meeting the recommended threshold and enhancing the reliability of comparisons. Given these practical constraints, the chosen sample size is considered adequate for an initial exploratory investigation and provides valuable insights into the study objectives. Participants were selected from the Hematology and Oncology Unit of the Pediatric Department at Menoufia University Hospitals, Egypt, over the period from December 2022 to December 2023. All patients received IVIG as part of their treatment. The research was carried out in collaboration with the Clinical Pathology, Medical Biochemistry, and Molecular Biology Departments at the Faculty of Medicine, Menoufia University. Ethical approval was obtained from the Menoufia University Research Ethics Committee (IRB number: 12/2018 CPATH 43), with obtaining written consent from the parents or legal guardians of all participants.
1. Inclusion and exclusion criteria
Pediatric cases confirmed to have acute primary ITP, aged between 1 to 18 years were involved. Primary ITP was defined as an isolated decrease in platelet count of less than 100×109/L, without any underlying cause [4-6]. Children with secondary causes of thrombocytopenia such as autoimmune disorders, drugs, lymphoproliferative disorders, vaccinations, and immunodeficiency syndromes were excluded from the study.
2. All participating children underwent the following evaluations
Acute primary ITP was clinically confirmed in all patients included in the study based on a comprehensive history-taking, which included details such as name, age, sex, and the history of the current complaint. This included the onset, duration, and manifestations of the condition, such as skin or mucous membrane bleeding, and its severity based on the grading system by Buchanan and Adix [14] Additionally, the history covered any associated symptoms such as fever, arthralgia, bony aches, weight loss, or any drug intake. A past medical history was taken, including any previous similar conditions, surgical operations, and family history of similar conditions. Following history-taking, a thorough physical examination was conducted, including a general examination of the patient's appearance, mucous membranes, and limbs. Skin examination focused on signs of petechiae, purpura, and ecchymosis. The lymph nodes were also examined, along with an abdominal examination and a systemic examination for any abnormalities.
Two peripheral blood (PB) samples were obtained from each participating child. The first sample was utilized for conducting a complete blood count (CBC) and preparing a PB smear, both analyzed using automated Sysmex XN-1000 Hematology Analyzer (Sysmex America, Inc., USA). The second sample was designated for evaluating the relative expression levels of the Bak and Bcl-Xl genes. After IVIG treatment, 2 additional blood samples were withdrawn from each patient to reevaluate the effects of the treatment on both CBC parameters and the relative gene expression of Bak and Bcl-Xl genes.
3. Molecular genetic study
Gene expression levels of Bak and Bcl-Xl were assessed using reverse transcription quantitative polymerase chain reaction (RT-qPCR). For this purpose, 2 mL of peripheral venous blood were drawn into EDTA-containing vacuum tubes from both ITP patients and healthy controls. The samples were centrifuged at 850 rpm for 10 minutes to separate the platelet-rich plasma, which was then transferred into Eppendorf tubes and stored at -20°C pending analysis.
1) Principles
Following the manufacturer’s protocol, RNA was totally extracted from supernatant plasma using the miRNeasy Mini Kit (Catalog no. 217004, Qiagen, Germany). The concentration and purity of the extracted RNA were assessed using the Nanophotometer N60 (IMPLEN, Germany) with one microliter of the RNA sample. Reverse transcription and RT-qPCR were performed sequentially in the same tube using the One Step RT-PCR Kit (Catalog no. 210212, Qiagen). The ABI 7500 real-time PCR detection system (Applied Biosystems, USA) was used to detect the relative gene expression. The thermal cycling protocol included an initial reverse transcription step at 50°C for 30 minutes, followed by PCR activation at 95°C for 15 minutes. This was succeeded by 40 amplification cycles consisting of denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 1 minute. Finally, an extension phase was carried out at 72°C for 10 minutes.
2) Primers sequences
(1) The primers for Bak gene were: Forward: 5′-TGAGTACTTCACCAAGATTCA-3′Reverse: 5′-AGTCAGGCCATGCTGGTAGAC-3′
(2) The primers for Bcl-Xl gene were: Forward: 5′-TTACCTGAATGACCACCTA-3′Reverse: 5′-ATTTCCGACTGAAGAGTGA-3′
(3) The primers 5S ribosomal RNA gene (5S rRNA) were: Forward: 5′-TACGGCCATACCACCCTGAA-3′Reverse: 5′-TAACCAGGCCCGACCCTGCT-3′ which was used as the reference gene for standardizing Bak and Bcl-Xl gene expression levels.
3) Calculation of relative gene expression
The relative expression levels of Bak and Bcl-Xl were calculated using the 2-ΔΔCt method [ΔΔCt = ΔCt (sample) – ΔCt (control)], where ΔCt = Ct (target gene) – Ct (reference gene), and Ct indicate the threshold cycle.
4. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics ver. 22.0 (IBM Co., USA). Quantitative data were presented as mean±standard deviation, range (minimum-maximum), and median (interquartile range), while qualitative data were expressed as frequency (n) and percentages (%).
The chi-square test was utilized to examine associations between qualitative variables. Student t test was employed to compare quantitative data between the 2 groups when the data normally distributed. Data from 2 independent groups with nonnormal distribution were compared using the Mann-Whitney U test. The Wilcoxon signed-rank test was employed for paired comparisons of nonnormally distributed quantitative variables.
Pearson correlation coefficient was utilized to evaluate the relationship between quantitative variables when they followed a normal distribution, whereas Spearman correlation coefficient was employed for variables that were not normally distributed. Statistical significance was defined as a P value less than 0.05, with values below 0.001 indicating a highly significant result in 2-tailed tests.
Results
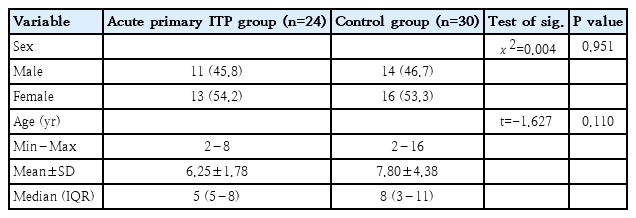
A total of 54 children participated in this study, who were classified into 2 groups: 24 patients with acute primary ITP (11 boys and 13 girls, having a mean age of 6.25±1.78 years) and 30 children in the healthy controls (14 boys and 16 girls, with a mean age of 7.80±4.38 years). Patients and controls were adequately matched in age and sex distribution (Table 1).
Before treatment, all patients experienced skin bleeding, which decreased to 16.7% following treatment. Oral bleeding was observed in 62.5% of cases prior to treatment, but resolved completely afterward. Epistaxis was reported in 45.8% of patients before treatment and was absent posttreatment. Additionally, 2 patients presented with GIT bleeding, specifically melena (Fig. 1).
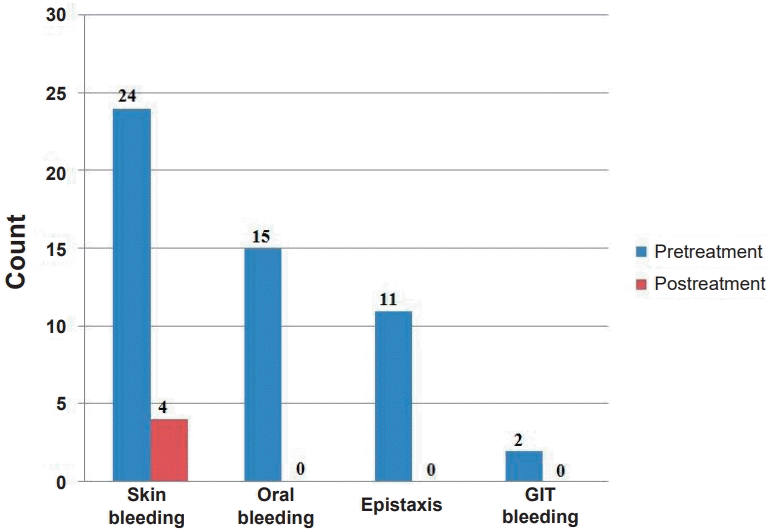
Number of patients presenting with skin bleeding, oral bleeding, epistaxis, or gastrointestinal (GIT) bleeding before versus after treatment.
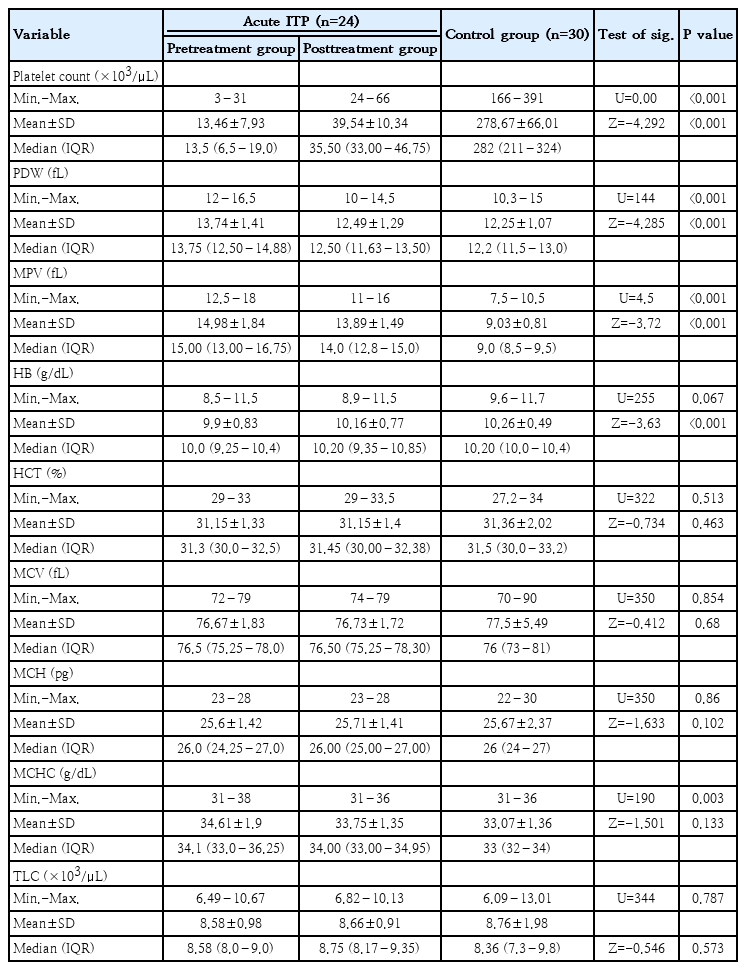
Platelet count was significantly lower in patients in reference to control group (P<0.001), whereas both platelet distribution width (PDW) and mean platelet volume (MPV) were significantly higher in patients (P<0.001). No significant differences were observed in other CBC parameters between the 2 groups, except for mean corpuscular hemoglobin concentration (MCHC), which was significantly higher in patients (P=0.003). Following treatment, there were significant increase in both platelet count and hemoglobin levels, while PDW and MPV significantly decreased (P<0.001) (Table 2).
Comparing the studied groups with regard to platelet Bcl2 apoptotic markers revealed the following: In the pretreatment patient group, the Bak gene expression level and Bak/Bcl-Xl expression ratio were significantly higher than in the control group (P=0.001 and P<0.001, respectively). However, Bcl-Xl gene expression was significantly lower than in the control group (P=0.029), as shown in Table 3 and Fig. 2A–C. Furthermore, after treatment, there was a significant decrease in the expression of both Bak and the Bak/Bcl-Xl ratio compared to the pretreatment level (P<0.001and P=0.001, respectively). On the other hand, Bcl-Xl gene expression was significantly increased posttreatment (P<0.001).
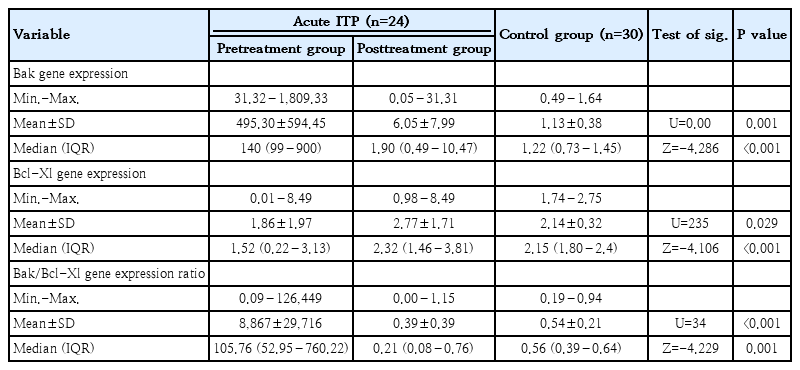
Bak gene expression level, Bcl-Xl gene expression level, and Bak/Bcl-Xl gene expression ratio of acute ITP versus control
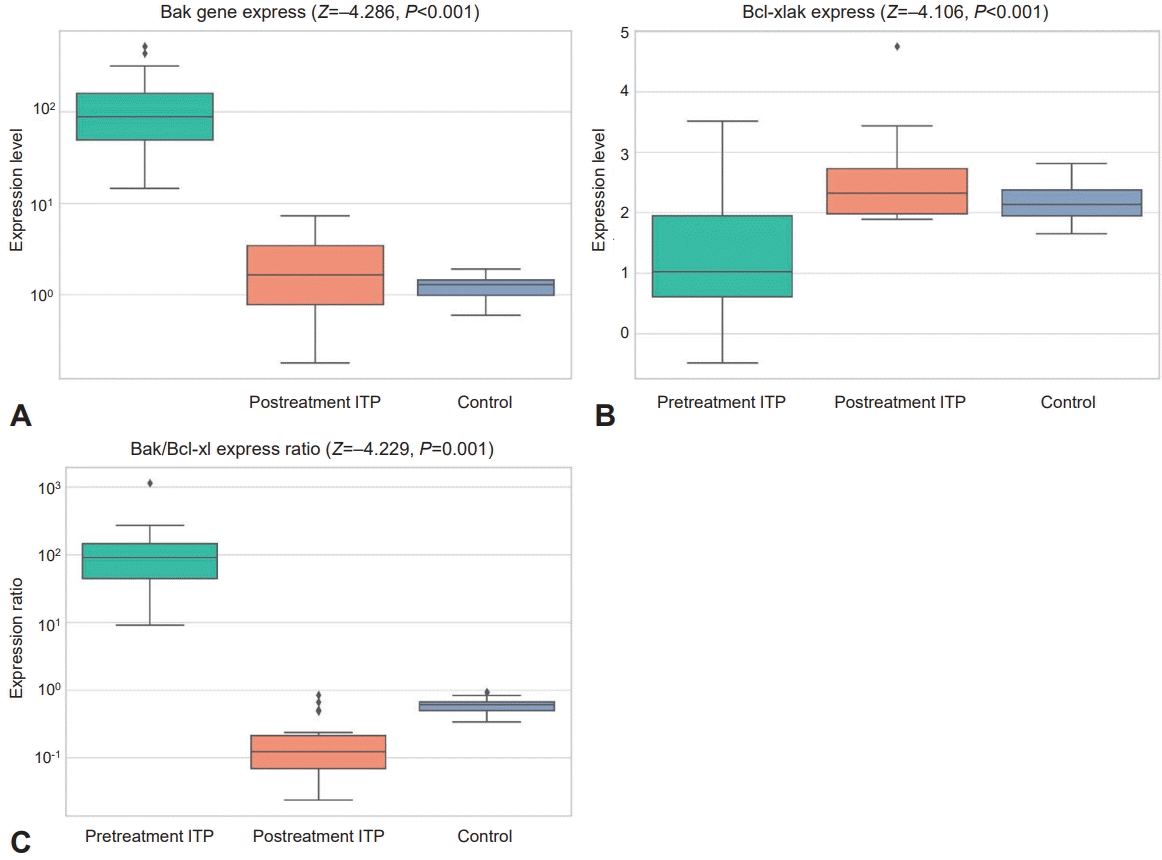
Differential expressions of Bak and Bcl-Xl genes in pediatric patients with ITP pre- versus posttreatment compared to healthy controls. (A) Bak gene expression. (B) Bcl-Xl gene expression. (C) Bak/Bcl-Xl expression ratio. ITP, immune thrombocytopenia (Z, Wilcoxon signed ranks test for pre- versus posttreatment ITP groups; P<0.05, significant; P<0.001, highly significant)
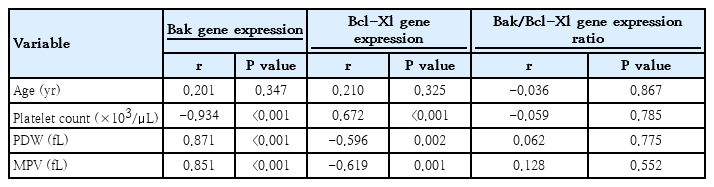
Bak gene expression displayed an inverse correlation with platelet count (P<0.001) and displayed a positive correlation with PDW (P<0.001) and MPV (P<0.001). In contrast, Bcl-Xl gene expression demonstrated a positive correlation with platelet count (P<0.001) and negative correlations with PDW (P=0.002) and MPV (P=0.001) (Table 4).
Discussion
Acute primary pediatric ITP is an autoimmunity disorder defined by standalone thrombocytopenia, with a platelet number of down to 100×109/L, occurring with no identifiable cause in children aged 1 to 18 years. Depending on the duration of the disease, ITP can be classified as ‘acute’ when thrombocytopenia resolves before 6 months with more than three quarters recovering spontaneously. Most ITP children present with minimal symptoms, including bruising, petechiae and or mild epistaxis [15].
Accelerated peripheral platelet breakdown and diminished platelet production are the 2 main pathogenic mechanisms underlying ITP [16]. The primary cause of enhanced platelet breakdown is the targeting of platelet membrane glycoproteins by IgG autoantibodies [17]. Despite being anucleate, platelets undergo processes similar to apoptosis, which is the primary pathophysiological mechanism regulating their lifespan [18,19].
This study investigated the relation between thrombocytopenia in pediatric acute ITP and platelet apoptosis by assessing Bak and Bcl-Xl expression. Results were compared with healthy controls, and post-IVIG treatment changes were evaluated in the same patients.
This study includes 24 acute primary ITP patients and 30 healthy controls with no significant difference between the groups regarding age and sex with equal sex distribution in both groups. In accordance, Grimes et al. [20], reported that, mean age of diagnosis among those with acute ITP was 5.5 years with equal sex distribution in the entire cohort. Also, Makis et al. [21] found that newly diagosed ITP cases had a balanced male-to-female ratio. While Sedeek et al. [22] studied 40 pediatric patients with ITP and found that boys were affected more than girls.
Clinically, ITP patients typically present with skin and mucosal bleeding. In our cohort, skin bleeding was the predominant clinical feature, followed by oral bleeding, epistaxis, and less frequently, GIT bleeding. After IVIG treatment, bleeding significantly subsided in majority cases with acute ITP. This aligns with the results observed by Al-Zuhairy [23], he reported that bruising and petechiae were the commonest clinical features in pediatric ITP patients, followed by mucosal bleeding. Additionally, Makis et al. [21] found that mucosal bleeding was observed in 70% of new ITP cases, 2 patients had conjunctival hemorrhage, and one case involved menorrhagia. Yahia et al. [24], observed that in newly diagnosed ITP patients, clinical symptoms subsided following IVIG treatment as platelet counts increased.
Intrinsic and extrinsic pathways are the 2 primary apoptotic pathways in platelets. These pathways control the platelet programmed death [9]. The first pathway (intrinsic) is controlled by BCL2 proteins, that include antiapoptotic proteins (like Bcl-Xl) and apoptotic proteins (e.g., Bak, BAD & Bax).These proteins can trigger alterations in the extrinsic membrane of mitochondria, causing the discharge of cytochrome c out in cytoplasm [12]. According to our study, pretreatment acute ITP group showed increased expression of proapoptotic marker Bak which was significantly higher than all other groups. While Bcl-Xl expression was significantly lower than control group.
As regards Bak/Bcl-Xl expression ratio, it was significantly increased in pretreatment acute ITP more than group of healthy controls. This indicates that acute phase showed an increased tendency towards apoptosis. With these findings Winkler et al. [25] observed heightened indicators of apoptosis in platelets, namely caspase-3 activation, phosphatidylserine exposure, and production of microparticles. Also Levy-Mendelovich et al. [26] revealed significantly higher apoptotic proteins (BIM, CD40, IGFBP2, P21, and SMAC) after incubating platelets with sera from acute ITP children at diagnosis, in comparison with those incubated with sera from chronic patients.
ITP treated primarily with corticosteroids and IVIG. IVIG is often the preferred option, as it not only raises platelet counts but also inhibits the reticulo-endothelial system through Fc-receptor blockade and modulates platelet apoptosis [27]. In our results, the post-IVIG treatment acute ITP group patients showed decreased expression of proapoptotic marker Bak which was significantly lower than pretreatment acute ITP group and control group. While the expression of antiapoptotic marker Bcl-Xl was significantly higher than pretreatment acute ITP group. This indicates decreased apoptosis after treatment with IVIG. This comes in accordance with Speer and Schmugge [28] who investigated apoptotic markers in ITP, they found that IVIG treatment reduced apoptotic-like events and improved low platelet counts in a murine ITP model. Also, Yahia et al. [24] revealed elevated BCL2 levels in newly diagnosed ITP patients following IVIG therapy. Similarly, Winkler et al. [25] observed an improvement in platelet apoptosis markers after IVIG administration. This observed reduction in apoptotic tendency following IVIG therapy highlights its efficacy in restoring platelet survival. Early administration of IVIG in acute ITP cases may help prevent severe thrombocytopenia and reduce bleeding risks.
According this study, there was significant decrease in platelet count in acute group prior to treatment than after treatment group and control group, while the control group was significantly higher than all the other groups as regards platelets count. This aligns with the results observed by Al-Zuhairy [23], who reported a mean platelet count of 13.200×103/L in newly diagnosed cases of childhood primary ITP. Similarly, Yahia et al. [24] observed a significant rise in platelet count following IVIG treatment in newly diagnosed ITP patients, in agreement with our results.
Regarding CBC parameters other than platelet count, it had been demonstrated that no notable difference was observed between the acute ITP group and the control group across all parameters except for PDW, MPV and MCHC which were significantly high in acute ITP more than the group of healthy controls indicating increased platelet turnover or activation. According to Pietras and Pearson-Shaver [29], the leucocytes count, Hb levels, indices of red blood cells, and differential in ITP are typically within ranges, with only a reduced platelet count [30].
Bak gene expression showed an inverse correlation with platelet count, indicating that increased Bak expression may contribute to enhanced platelet apoptosis, leading to thrombocytopenia. Additionally, its positive correlation with platelet indices such as PDW and MPV suggests that increased platelet turnover or activation may accompany higher Bak expression. Conversely, Bcl-Xl displayed a protective role, as evidenced by its positive correlation with platelet count and negative association with platelet indices and bleeding symptoms. Higher Bcl-Xl expression likely enhances platelet survival, maintaining a stable platelet count and reducing variability in platelet size and activation markers and bleeding manifestations.
Overall, these findings highlight the critical balance between proapoptotic (Bak) and antiapoptotic (Bcl-Xl) mechanisms in maintaining platelet homeostasis. Disruption of this balance may increase susceptibility to thrombocytopenia and bleeding disorders. Future research may focus on therapeutic strategies targeting these apoptotic pathways to modulate platelet lifespan in bleeding disorders or thrombocytopenia.
This study has several limitations. The relatively small sample size and its single-center design may restrict the generalizability of the findings to broader populations. Additionally, the analysis was limited to short-term outcomes following IVIG therapy, without evaluation of long-term effects or potential relapse. The investigation focused solely on the gene expression of Bak and Bcl-Xl, lacking supportive functional assays or protein-level validation. Furthermore, the study did not include a comparative analysis with other standard treatment options such as corticosteroids, which could have provided deeper insight into differing therapeutic mechanisms.
In conclusion, we conclude increased tendency towards apoptosis in acute primary ITP as Bak expression and Bak/ Bcl-Xl expression ratio are significantly higher than control group and reduced expression of the antiapoptotic Bcl-Xl gene. This tendency toward apoptosis decreased significantly after treatment with IVIG with increased platelet count and bleeding cessation.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: AZB, IAA, HHE; Data curation: AZB, DFSENZ, HHE; Formal analysis: IAA, HHE; Funding acquisition: HHE; Methodology: AZB, IAA, DFSENZ, HHE; Project administration: AZB, HHE; Visualization: AZB, IAA, HHE; Writing - original draft: AZB, SMEH, DFSENZ, HHE; Writing - review & editing: SHK, MAE, SMEH, HHE