Evolving treatment strategies for invasive Streptococcus pyogenes in children in the postpandemic era
Article information
Abstract
Background
Streptococcus pyogenes (group A Streptococcus [GAS]) is a common cause of bacterial pharyngitis and skin infections in children that can lead to severe and invasive GAS (iGAS) infections. The sudden acute respiratory syndrome coronavirus 2 pandemic coincided with an increase in iGAS cases, with emerging serotypes and risk factors like age, reduced postpandemic immunity, and viral coinfections. The treatment of iGAS with clindamycin and intravenous immunoglobulins (IVIG) is not well standardized, and pediatric data are limited. Linezolid is being explored as an alternative to clindamycin, although further research is required.
Purpose
This study aimed to evaluate the treatment of iGAS in hospitalized children with focus on the effectiveness of standard treatments and the role of alternative interventions in cases of treatment failure, including the use of linezolid or severe infections. Additionally, this study sought to identify the potential risk factors for pediatric intensive care unit (PICU) admission.
Methods
A retrospective observational study was conducted in children aged <18 years admitted to Meyer University Children's Hospital (September 2022 to September 2024) with confirmed or probable iGAS. Their anonymized general information, symptoms, laboratory test results, microbiological test results, coinfections, radiological examination results, antibiotic and nonantibiotic therapies, discharge information, and outcomes were collected.
Results
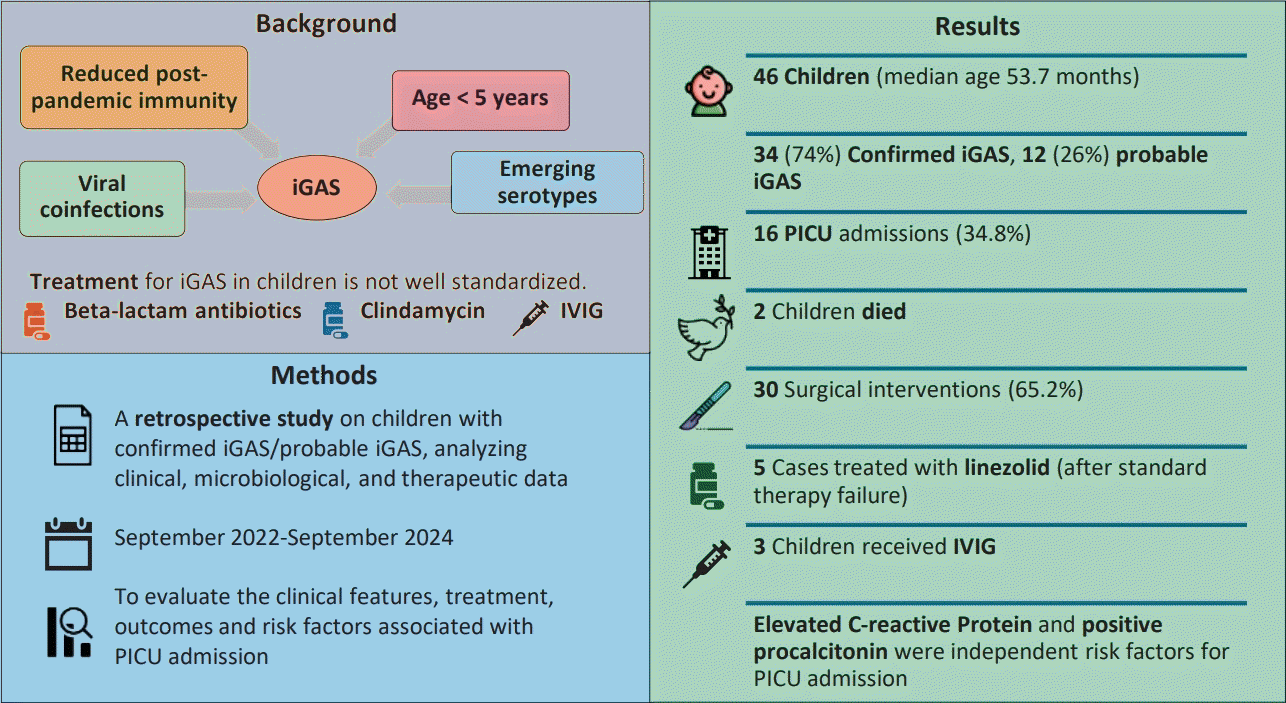
Forty-six children with confirmed/probable iGAS (median age, 53.7 months) were included. Of them, 34 (73.9%) had confirmed iGAS and 12 (26.1%) had probable iGAS. Sixteen children (34.8%) with iGAS were admitted to the PICU; of them, 2 died. All children received beta-lactam antibiotics; in 5 cases (10.9%), linezolid was administered after beta-lactam and clindamycin therapy failure. Thirty patients (65.2%) underwent surgery. Of the study population, 22% had preexisting conditions and 17% had viral respiratory coinfections. Elevated C-reactive protein and procalcitonin levels were independent risk factors for PICU admission. IVIG administered to 3 patients had varying outcomes.
Conclusion
Our findings highlight how treatment strategies and disease patterns have shifted in the postpandemic period. Pneumonia with a parapneumonic abscess or empyema has emerged as the most frequent clinical presentation, with nearly half of such cases requiring second-line linezolid therapy. Linezolid may be a valuable treatment alternative after beta-lactam and clindamycin failure. IVIG has been used in severe cases but often late, warranting further investigation into its optimal application.
Key message
Question: What are the roles of linezolid, intravenous immunoglobulin (IVIG), and corticosteroids in pediatric invasive group A streptococcal infection (iGAS)? Can any improve outcomes beyond beta-lactams and clindamycin?
Finding: Two of 46 patients with iGAS died. Nearly all received beta-lactams plus clindamycin. Linezolid was effective in refractory cases. IVIG and corticosteroids had variable efficacies.
Meaning: Linezolid may be valuable in refractory cases. IVIG may be considered in severe presentations. The role of corticosteroids remains less clearly defined.
Graphical abstract. iGAS, invasive group A streptococcal infection; IVIG, intravenous immunoglobulin; PICU, pediatric intensive care unit.
Introduction
Streptococcus pyogenes (group A Streptococcus, GAS) is one of the most common causes of bacterial pharyngitis and skin infections in children. Most S. pyogenes infections are benign and self-limiting; however, in a modest percentage of cases, GAS infection is associated with severe or invasive clinical manifestations (invasive group A streptococcal infection, iGAS). In these cases, the infection can rapidly evolve into a severe systemic disease due to an overwhelming inflammatory response, with a mortality rate ranging from 0% to 14% [1].
Historically, until 2022, the reported incidence of iGAS in Western countries was low, ranging between 1.5 and 3.8 cases per 100,000 per year, and was higher in children under 1 year of age [2,3]. By the end of the severe acute respiratory syndrome coronavirus 2 pandemic, when prevention measures were lifted, an increase in the global incidence of iGAS cases was observed: on December 12, 2022, the World Health Organization reported an increase in invasive GAS infections in several European countries [4]; in February 2023, a similar trend was observed by the Centers for Disease Control and Prevention in the United States [5].
The most common clinical presentations of iGAS include pneumonia complicated by empyema or parapneumonic effusion, followed by soft tissue infection, bacteremia without a specific focus, and streptococcal toxic shock syndrome (STSS). Cases of complicated pneumonia occurred without a seasonal pattern in children under 5 years of age, with rapid clinical deterioration leading to longer hospital stays, high rates of admission to pediatric intensive care unit (PICU), surgical management, and high mortality rates [6].
Several studies have identified 4 potential risk factors for the recent increase in the incidence of iGAS: age under 5 years, reduced training immunity in the postpandemic period after lifting prevention measures, viral respiratory coinfections primarily caused by respiratory syncytial virus (RSV), influenza, metapneumovirus, and varicella [6]. Additionally, emerging serotypes such as emm1 and emm12 and highly virulent strains such as M1UK and M1DK, which some authors report as spreading more widely, could be partly responsible for the increase in iGAS incidence [7,8]. However, the diversity of emm serotypes isolated in different countries suggests that a single variant is not the predominant cause of the phenomenon [6].
While beta-lactams remain the cornerstone of iGAS treatment, limited pediatric data are available to support the use of adjunctive therapies or second-line agents. This uncertainty underscores the need for updated clinical data to guide treatment choices, particularly in severe or refractory cases.
To evaluate the effectiveness of standard treatments and the role of alternative interventions in cases of treatment failure in children hospitalized for iGAS in a tertiary pediatric hospital in the postpandemic period, we conducted a retrospective observational study. Additionally, we aimed to identify potential risk factors associated with transfer to PICU.
Methods
A retrospective observational study was conducted on data regarding children (under 18 years of age) admitted to the Meyer University Pediatric Hospital from September 1, 2022, to September 30, 2024.
Inclusion criteria were: age <18 years; hospital admission; diagnosis of confirmed iGAS or probable iGAS. The exclusion criterion was age >18 years.
Hospitalization data and medical records of eligible patients (including patient information/history, demographic characteristics, and baseline assessments) were reviewed and subsequently anonymously entered into an electronic database.
The collected data include: (1) general data: gender, age, place of birth, nationality/place of birth of parents, any comorbidities or immunodeficiency, surgical procedures in the month prior to admission, use of antibiotic therapy or nonsteroidal anti-inflammatory drugs in the 2 weeks prior to admission, possible travel abroad; (2) symptoms and clinical presentation at onset; (3) laboratory tests at admission, including leukocyte count, neutrophilia, C-reactive protein (CRP), procalcitonin (PCT); urinalysis; (4) results of the rapid strep antigen test; (5) microbiological results such as blood cultures, polymerase chain reaction (PCR) tests on blood, cultures on other biological materials, PCR on other biological materials, results of antibiotic susceptibility tests; (6) viral or bacterial coinfections; (7) results of radiological examinations (e.g., ultrasound, x-ray, computed tomography, magnetic resonance imaging, and other types of imaging); (8) medical therapies: type of antibiotic therapy, duration, route of administration, doses, use of glucocorticoids or intravenous immunoglobulins, adverse events to therapy; (9) nonantimicrobial therapies such as surgery, invasive ventilatory support, or extracorporeal circulation; (10) discharge information: duration of hospital stay, admission to PICU, discharge diagnosis, possible outcomes or deaths.
Patients were divided into: children admitted to PICU and children not admitted to PICU, and children with confirmed iGAS and children with probable iGAS.
1. Definitions
A case of confirmed iGAS was defined as the detection of S. pyogenes by culture or accredited molecular methods PCR in a normally sterile body site, including blood, cerebrospinal fluid, joint aspirate, pericardial-peritoneal-pleural fluid, bone, endometrium, deep tissue, or deep abscess in the operating room [9].
A case of probable iGAS was defined as the isolation of GAS from a normally non-sterile site such as pharynx, sputum, or wounds, associated with a severe clinical presentation, such as STSS, necrotizing fasciitis (NF), pneumonia, septic arthritis, meningitis, peritonitis, osteomyelitis, myositis, sepsis, and septic shock [9].
Sepsis was defined according to the Phoenix Sepsis Score as life-threatening organ dysfunction associated with infection. The score analyzes 4 systems (respiratory, cardiovascular, coagulation, neurological) and defines sepsis as the presence of a score ≥2 and septic shock as the presence of sepsis and at least 1 point in the cardiovascular component of the score in children with suspected or confirmed infection [10].
2. Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR). Categorical variables were expressed as numbers and percentages. For categorical variables, we performed the chi-square or Fisher test; for continuous variables, medians between groups were compared using the Wilcoxon Mann-Whitney U test. Possible risk factors for PICU admission were investigated using univariate and multivariate logistic regression analysis. Factors were included in the multivariate analysis if they were significant (P<0.05) in the univariate analysis. Stata14 (StataLLC, USA) for Windows was used for the analyses.
3. Ethical statement
This study was approved by the National Ethics Committee for Research Conducted by Public Research Institutions and Other National Public Bodies (CEN). The approval was granted under protocol number 0025795.
Results
Forty-six children with confirmed/probable iGAS were included (median age, 53.66 months; IQR, 29.68–88.49 months; 27 males [58.70%] and 19 females [41.30%]): 34 confirmed iGAS (73.91%) and 12 probable iGAS (26.09%). Sixteen patients (34.78%) with iGAS were admitted to PICU, 2 children died. Clinical characteristics and laboratory test results of the patients are reported in Table 1.
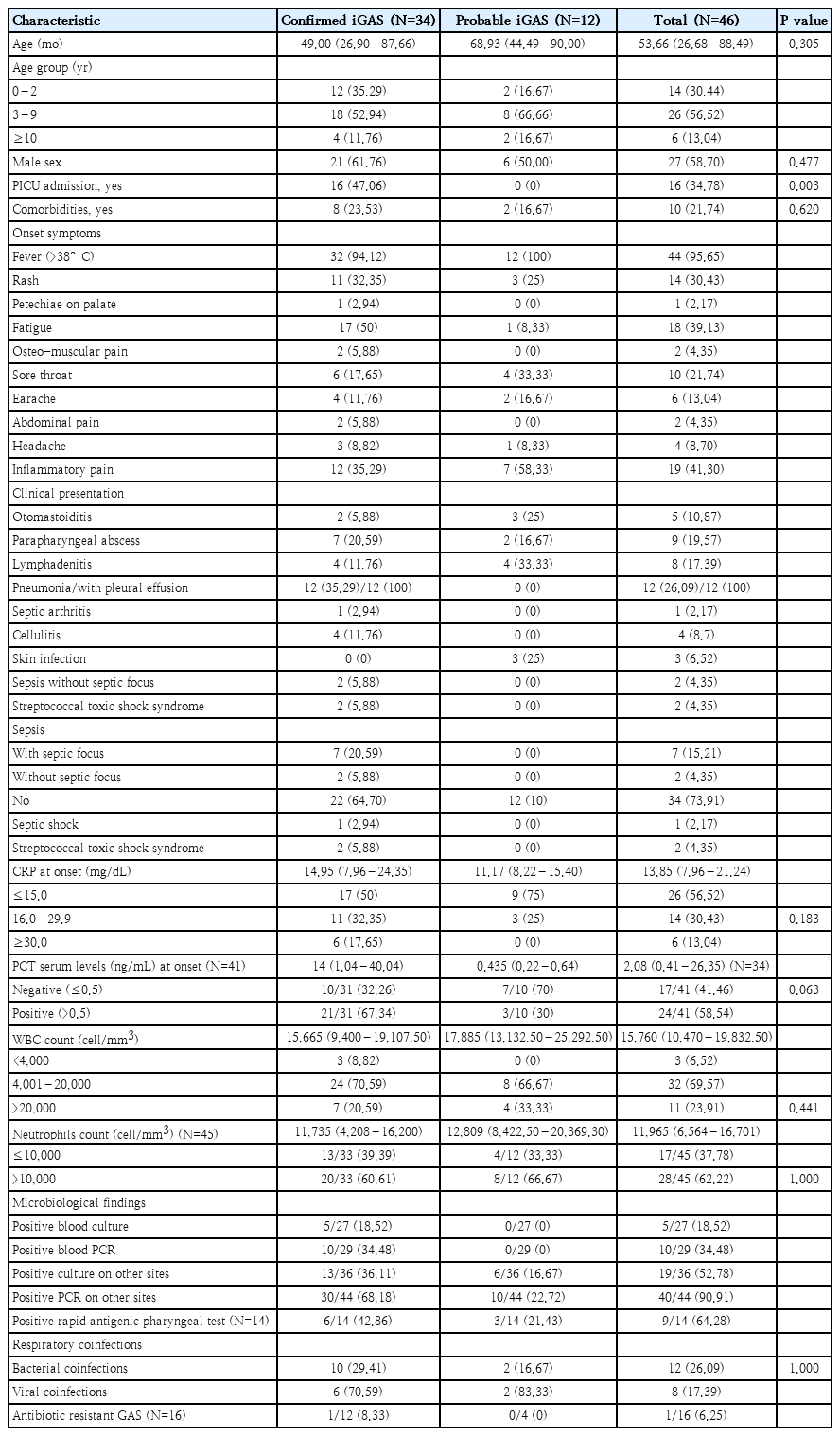
Clinical characteristics and microbiological results of children with confirmed versus probable iGAS
Ten children had preexisting comorbidities (1 congenital heart disease, 2 epilepsy, 1 genetic syndrome with xq28 duplication, 1 therapy-induced immunosoppression in juvenile idiopatic arthritis, 3 respiratory diseases, 1 achondroplasia, 1 cognitive and psychomotor development delay). Clinical details and outcomes of these children are reported in supplementary material.
Among the 34 confirmed iGAS cases, there were 12 cases of pneumonia (35.29% of total iGAS cases), all with associated pleural effusion, 2 cases of STSS (5.88%), 2 cases of sepsis without septic focus (5.88%), 4 cases of cellulitis (11.76%), 1 case of septic arthritis (2.94%), 4 cases of lymphadenitis (11.76%), 7 cases of retropharyngeal/peritonsillar abscesses (20.59%), and 2 cases of otomastoiditis (5.88%) (Fig. 1). In children aged 0–2 years, the predominant clinical presentation was pneumonia, while in children older than 3 years, parapharyngeal abscess was most frequently observed (Fig. 2).
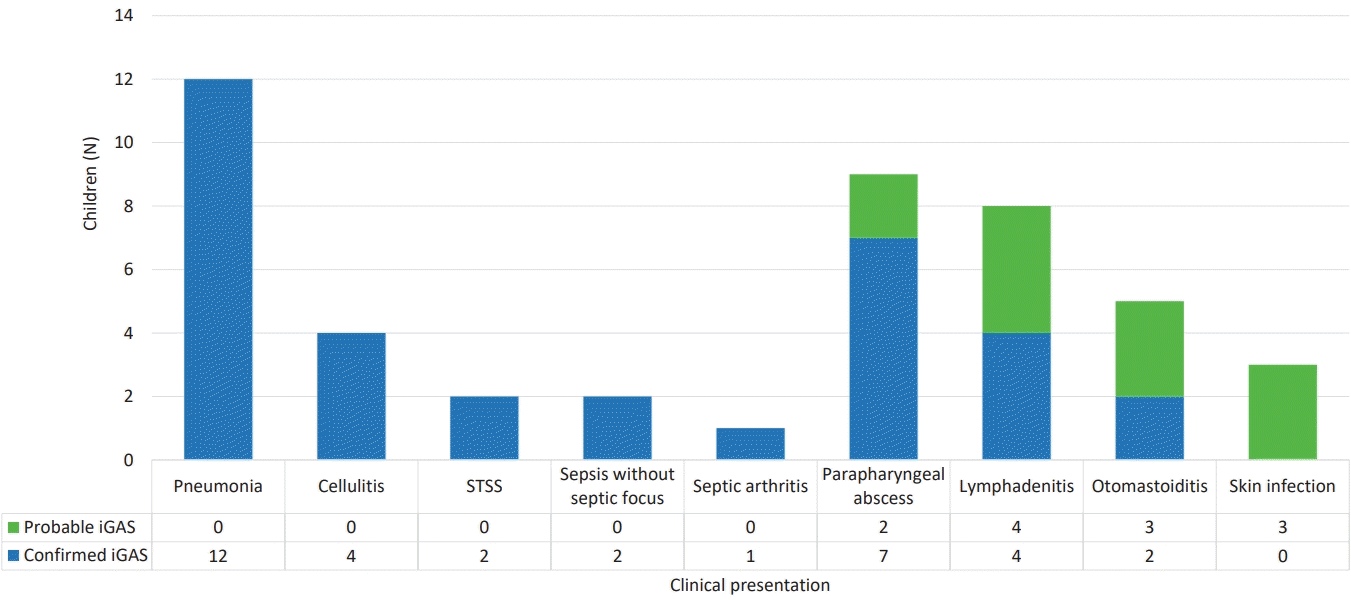
Clinical presentations of children with confirmed/probable iGAS. iGAS, invasive group A streptococcal infection; STSS, streptococcal toxic shock syndrome.
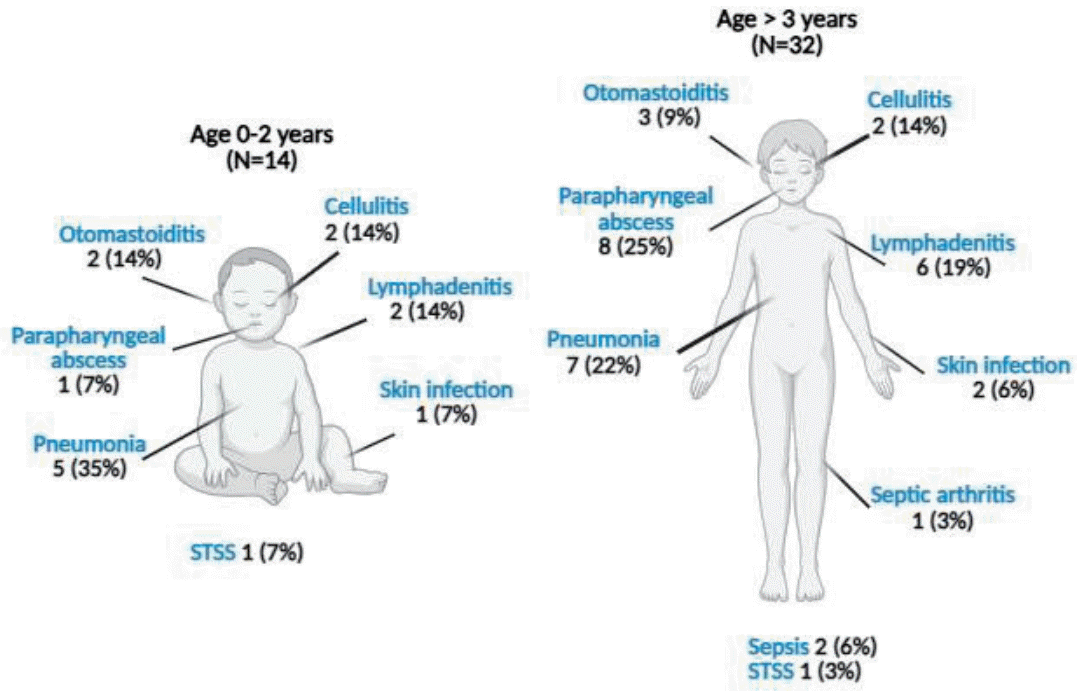
Clinical presentation by age group of children with confirmed/probable invasive group A streptococcal infection. STSS, streptococcal toxic shock syndrome. Created with Biorender.com.
Eight patients had viral respiratory coinfections (17.39%) bronand 12 (26.09%) had bacterial coinfections. Two of the 8 (25%) had more than one viral respiratory infection, as did 4 of 12 patients (33.33%) with more than one bacterial respiratory infection. Further details are shown in supplementary material.
Regarding microbiological investigations, 5 out of 27 blood cultures (18.52%) and 4 out of 7 pleural fluid cultures (57.14%) were positive; For all other materials (bronchoaspirate and bronchoalveolar lavage, synovial fluid, ocular, pharyngeal and skin swabs, abscess and lymph node drainage), 12 out of 20 cultures (60%) were positive. In blood, PCR was positive in 9 out of 16 cases (56.25%), while in pleural fluid it was positive in all analyzed samples (12 of 12, 100%); for all other materials, PCR was positive in 23 out of 24 samples (95.83%). Further details are shown in supplementary material.
Sixteen strains received antibiotic susceptibility test results, and among them, resistance was found in only 1 strain (6.25%), resistant to levofloxacin, trimethoprim/sulfamethoxazole, teicoplanin, and doxycycline.
The temporal trend of infections followed a seasonal pattern, with no cases during the summer and early autumn months, and an increase during the winter/spring period. The highest number of cases occurred in April 2024, with a total of 8 cases: 6 confirmed iGAS and 2 probable iGAS.
1. Treatment
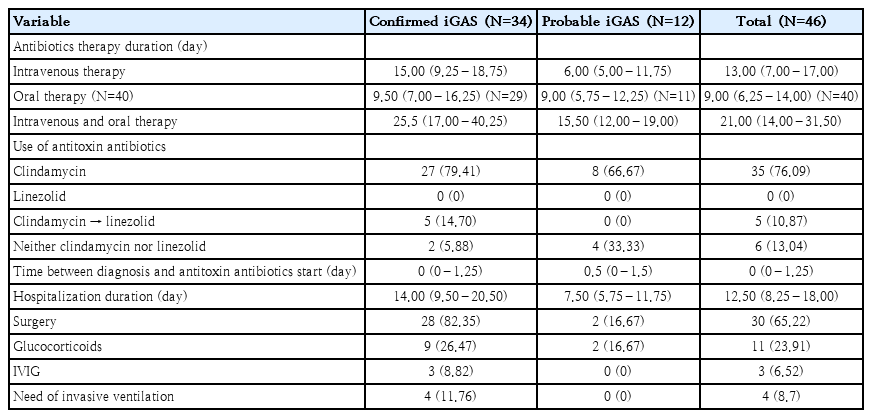
Data regarding antibiotic and nonantibiotic treatment are reported in Table 2.
All children received empirical treatment with beta-lactam antibiotics. Clindamycin was admistered in most cases (87%) as antitoxin agent. In 5 cases (10.87%), all presenting with pneumonia, clinical or radiological deterioration despite beta-lactam and clindamycin therapy prompted the introduction of linezolid. Specifically, 2 patients had persistent pleural effusion on imaging, while 3 continued to have fever and elevated inflammatory markers. Four of these 5 patients were admitted to PICU. No deaths occurred in this subgroup. Clinical outcomes according to antibiotic regimen are illustrated in Fig. 3, which compares PICU admission and other outcomes across 3 treatment groups. Linezolid was introduced only in patients with treatment failure and more severe disease, reflected by higher PICU admission rates in that group.
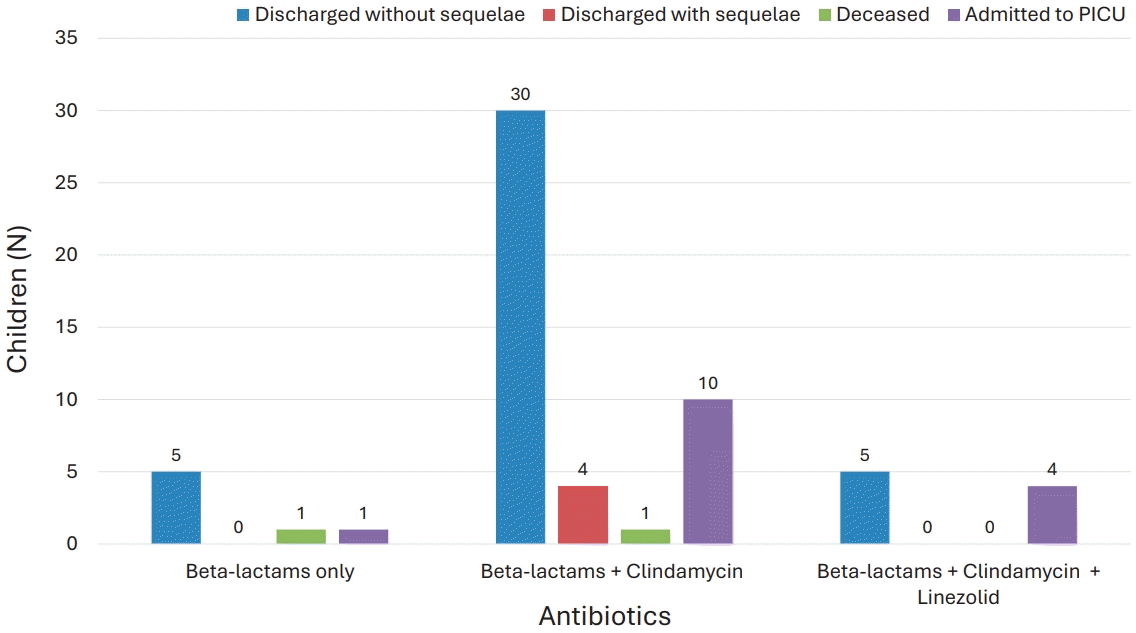
Antibiotic therapy adopted and corresponding outcomes in children with invasive group A streptococcal infection. Three children currently under radiological follow-up without clinical symptoms were not classified as having sequelae. PICU, pediatric intensive care unit.
Thirty children (65.22%) underwent surgical procedures, including: 11 (23.91%) thoracentesis with pleural drain placement, of which 4 (8.69%) were accompanied by lung debridement procedures using video-assisted thoracoscopic technique, 13 (28.26%) abscess drainage, 3 (6.52%) mastoidectomies, 1 (2.17%) arthrocentesis, 1 (2.17%) neurosurgery, and 1 (2.17%) functional endoscopic sinus surgery and orbital decompression.
2. Outcomes
Four children (8.69%) were discharged with infection-related sequelae, 2 children died, and 30 were discharged without complications.
Two children had minimal palpebral asymmetry, and 1 child presented elevation deficit and vertical diplopia, 1 child developed arterial hypertension following cardiac damage caused by sepsis and is currently on beta-blocker therapy. Three children are currently in radiological follow-up at the hospital center.
Two children died due to STSS. Both children developed critical multisystem failure, including respiratory failure requiring mechanical ventilation. GAS was identified in blood, respiratory secretions (pharyngeal swab and bronchoaspirate), and pleural fluid. Both received broad-spectrum antibiotics and IVIG. Steroids were not used. None of these fatal cases were treated with linezolid: one child received beta-lactam antibiotics and clindamycin, while the other was treated with beta-lactam antibiotics along with other antimicrobial agents.
3. Factors associated with PICU admission
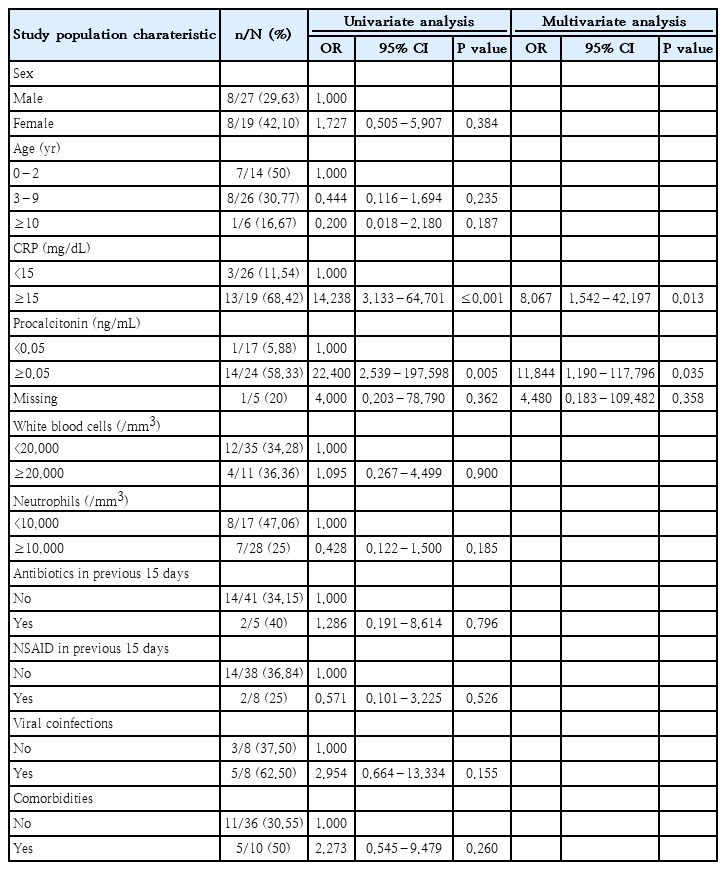
A univariate analysis (Table 3) was performed to evaluate factors potentially associated with PICU admission. Among these, positive serum PCT levels at onset (≥0.05 ng/mL) (odds ratio [OR], 22.400; P=0.005) and serum CRP levels ≥15 mg/dL (OR, 14.238; P=0.013) were significantly associated with PICU admissions. These 2 factors were subsequently analyzed in a multivariate analysis, which confirmed them as independent risk factors for PICU admission.
Discussion
In this monocentric retrospective observational study, we analyzed the clinical characteristics and therapeutic approach of 46 children hospitalized for confirmed iGAS (34 of 46, 73.91%) and probable iGAS (12 of 46, 26.09%) over 2 years. Among these children, 1 of 4 developed sepsis and were transferred to PICU. Two children died, with an observed fatality rate of 4.35%. Twenty-two percent of the children had preexisting conditions and 17% had a viral respiratory coinfection. Numerous studies suggest that iGAS cases are more likely to occur in conjunction with or following viral infections, especially those caused by RSV, influenza, varicella, and metapneumovirus [11,12]. In our study, varicella coinfection was not observed, likely due to the high vaccination coverage in Italy. The observed clinical manifestations align with data reported in the literature: 1 in 4 patients had pneumonia, all cases associated with pleural effusion. The therapeutic approach followed established empirical protocols: all children received initial antibiotic therapy with beta-lactams.
Although GAS is generally susceptible to beta-lactam antibiotics, many clinicians also prescribe antitoxin agents such as clindamycin, especially in cases of STSS or NF. Clindamycin acts by inhibiting protein synthesis and thereby reducing toxin production. Its use is supported by several adult and mixed-age observational studies, which suggest improved outcomes when used in combination with beta-lactams [13-15]. However, in the pediatric population, evidence remains scarce and somewhat conflicting, with small studies reporting both potential benefits and increased PICU admissions, possibly due to its selective use in more severe cases [1,16,17]. In our cohort, clindamycin was used in 87% of patients, consistent with these recommendations, according to disease severity or suspected toxin involvement. Nevertheless, in 5 cases (10.87%), clinical or radiological progression despite beta-lactam plus clindamycin therapy prompted a switch to linezolid. This highlights the importance of having effective second-line alternatives, particularly in severe presentations such as pneumonia with persistent pleural effusion or nonresolving systemic inflammation. Several factors may explain the possible failure of clindamycin in certain cases. Although generally well tolerated in pediatric population [18]. clindamycin has poor penetration across the blood-brain barrier, limiting its utility in central nervous system infections [19]. Its bacteriostatic mechanism may be less effective in life-threatening conditions compared to bactericidal agents, though recent evidence has questioned this paradigm [20,21]. Moreover, the global spread of clindamycin-resistant GAS strains raises additional concerns [22-24]. In our study, although the overall rate of resistance was low (6.25%), these considerations support the need for alternative or adjunctive therapies in selected patients. Linezolid, a synthetic oxazolidinone, inhibits bacterial protein synthesis and has demonstrated antitoxin activity. Adult studies suggest that it may be noninferior to clindamycin in terms of clinical outcomes, with added benefits such as lower risk of Clostridium difficile infection and effective methicillin-resistant Staphylococcus aureus coverage [22-24]. Its excellent pulmonary penetration could be particularly advantageous in cases of iGAS pneumonia [25]. In our study, linezolid was well tolerated and no adverse events were reported. However, its use in pediatrics still raises some concerns due to limited safety data and its off-label status in several European countries, including Italy [23]. Reported adverse events in children include gastrointestinal symptoms, rash, liver enzyme alterations, and less commonly, myelosuppression or neurological complications [26,27]. While these events are typically mild and reversible, careful monitoring is advised, especially in prolonged treatments. It is important to note, however, that the small number of patients treated with linezolid and the retrospective nature of the study may have limited the detection of mild or delayed adverse effects.
Regarding treatment duration, current literature does not provide clear guidance. Some experts recommend a minimum of 5–7 days of intravenous antibiotic therapy, continued until the patient is afebrile, hemodynamically stable, and with negative blood cultures [28]. In our cohort, the median intravenous antibiotic duration was 13 days, consistent with these empirical recommendations. This reflects the clinical severity of iGAS cases and the need for a prolonged course of therapy, particularly in complicated infections such as empyema or abscesses.
As for nonantibiotic therapies, their use in our cohort was heterogeneous and more common in severely ill patients. IVIG was administered in 3 cases—2 with STSS and one with sepsis. All 3 had severe disease; unfortunately, the 2 children with STSS died. The role of IVIG in pediatric iGAS remains controversial. Although it is thought to neutralize streptococcal superantigens and modulate immune response, evidence of its efficacy in children is limited and inconsistent. Some retrospective studies have found no significant improvement in outcomes with IVIG administration, likely due to confounding by indication, as more severe cases were more likely to receive it [29,30]. Others have suggested that IVIG is underused, particularly among fatal cases, implying a potential benefit if administered earlier in the disease course [31]. Current guidelines provide only general recommendations. The American Academy of Pediatrics recommends IVIG as adjunctive therapy in moderate to severe cases of STSS or NF [32], while the Canadian Paediatric Society suggests considering IVIG early in critically ill patients or when there is no response to initial fluid therapy [33]. In our study, the small number of treated patients and the late administration in both fatal cases highlight the need to better define the timing and criteria for IVIG use in pediatric iGAS. In our cohort, corticosteroids were administered in 24% of children. In the literature, only a few cases have been described, and none have evaluated the impact of steroid therapy on clinical outcomes. Despite the small number of cases in our study, the adverse outcomes highlight the urgency of developing guidelines that define the optimal timing for IVIG and corticosteroid administration. Early and targeted administration may improve clinical outcomes and potentially prevent exitus.
In our statistical analysis, we found that elevated CRP levels (>15 mg/dL) and positive PCT values (>0.05 ng/mL) are independent risk factors for PICU admission in iGAS cases. This result not only confirms the role of PCT as an indicator of invasive disease, as reported by other studies conducted on children hospitalized for community-acquired pneumonia [34], but also suggests that it could represent an early marker of sepsis. PCT has become a frequently used biomarker in sepsis due to its relative specificity for bacterial infections compared to viral ones [35]. However, its measurement is not sufficiently sensitive or specific to be used as the sole diagnostic test for sepsis, but can provide valuable information when interpreted in combination with other clinical evaluations [35].
This study has limitations. Due to its retrospective nature, it may introduce bias and reduce the possibility of establishing definitive causal relationships. The limited number of cases could also compromise the statistical power of the results. Additionally, as the data are collected from a single center, they may not fully represent the variability in clinical practices and patient outcomes observed across different regions or healthcare settings.
In conclusion, our study provides important data on the clinical characteristics and therapeutic approach of iGAS in pediatrics. Our results emphasize that although GAS is often considered a common pathogen, iGAS can lead to severe clinical outcomes, including transfer to PICU and most critically, death. Among the clinical presentations observed, pneumonia complicated by parapneumonic abscess or empyema was the most frequent, affecting 1 in 4 children in our cohort. In nearly half of these cases, second-line therapy with linezolid was required, highlighting its potential role as an effective alternative in cases of beta-lactam and clindamycin treatment failure. Additionally, IVIG was used in severe cases, but often late in the disease course, including in the 2 fatal cases. Further studies are needed to assess its true impact in clinical practice and determine the optimal timing for administration. Ultimately, timely and appropriate treatment remains crucial to improving patient outcomes. Future research should focus on refining therapeutic protocols, particularly regarding the decision to replace clindamycin with linezolid and the potential benefits of more frequent IVIG and corticosteroid use.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: EC; Data curation: LB, MR; Formal analysis: GI, EV; Methodology: GI, LG, EC; Project administration: EC, LG; Visualization: LB, MR; Writing-original draft: LB; Writing - review & editing: LB, EC, EV