Cytokine profile of Post–cardiopulmonary bypass in children
Article information
Abstract
Background
Open cardiac surgery involving cardiopulmonary bypass (CPB) triggers a systemic inflammatory response that significantly affects clinical outcomes. However, the dynamics and specific roles of cytokine release after CPB in the pediatric population remain unclear.
Purpose
To evaluate the dynamics of cytokine levels and their association with low cardiac output syndrome (LCOS)-related outcomes.
Methods
A prospective observational cohort study was conducted of 32 children who underwent elective open cardiac surgery with CPB at Songklanagarind Hospital, Thailand. Levels of interleukin (IL)-1β, IL-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α were analyzed preoperatively and immediately (T0), 6, 12, and 24 hours after intensive care unit admission. LCOS-related outcomes were defined with at least two of the following criteria being met within 24 hours postoperative: clinical and laboratory parameters, vasopressor-inotropic score ≥20, ejection fraction <50% on echocardiography; and requirement for a serious postoperative intervention. Statistical analyses utilized linear mixed models and multivariate logistic regression to identify the independent predictors of LCOS.
Results
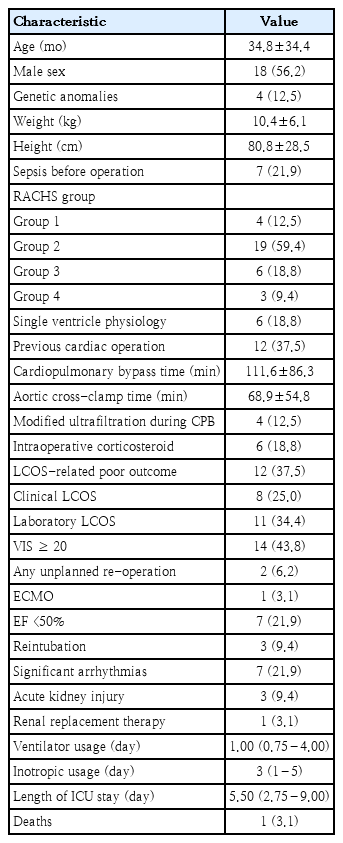
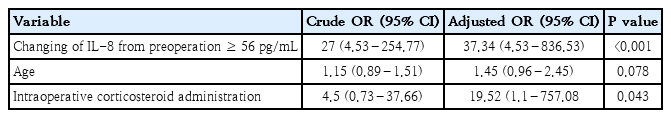
The mean patient age was 34.8±34.4 months; 56.2 % were male. Roughly one-third (37.5%) had a history of previous cardiac surgery, while one-quarter (28.3%) had a Risk Adjustment for Congenital Heart Surgery score ≥3. LCOS-related outcomes occurred in 37.5% of patients. IL-6, IL-8, and TNF-α levels differed significantly between patients with and without LCOS outcomes. An increase in IL-8 of >56 pg/mL from baseline to T0 showed the strongest association with LCOS (odds ratio, 37.34; 95% confidence interval, 4.53–836.53).
Conclusion
An elevated postoperative IL-8 level is a robust predictor of LCOS-related outcomes in pediatric patients undergoing cardiac surgery. These findings emphasize the importance of monitoring cytokine dynamics to guide interventions and improve patient outcomes.
Key message
Question: Can cytokine levels predict low cardiac output syndrome (LCOS) in children post–cardiopulmonary bypass?
Finding: Elevated interleukin (IL)-6, IL-8, and tumor necrosis factor-α levels were associated with LCOS, with an increase in IL-8 of >56 pg/mL from baseline to immediately postoperative being the strongest predictor.
Meaning: Monitoring immediately postoperative IL-8 levels may help identify pediatric patients at risk of LCOS, enabling timely interventions to improve outcomes.
Graphical abstract
Introduction
Open cardiac surgery in children, particularly procedures involving cardiopulmonary bypass (CPB), presents unique physiological challenges and triggers a systematic inflammatory response. The inflammatory mechanisms during CPB include: blood contact with the CPB circuit, ischemia-reperfusion injury, heparin-protamine interactions and surgical trauma. These factors exacerbate the activation of the complement cascade, release of endotoxins, and altered cytokine production, which can lead to various complications; including low cardiac output syndrome (LCOS) [1]. LCOS occurs in approximately 25% of children after cardiac surgery: typically manifesting 6-18 hours post operation [2].
Cytokines are mainly produced by monocytes, macrophages, lymphocytes and endothelial cells. In children, their immature immune system may react differently to the inflammatory stimuli induced by surgery and CPB when compared to adults. Previous studies have demonstrated that elevated levels of proinflammatory cytokines; such as interleukins (IL-6, IL-8) and tumor necrosis factor-alpha (TNF-α), are associated with postoperative morbidity and mortality [3]. Conversely, anti-inflammatory cytokines; such as IL-10 and IL-1ra, may also be elevated. This reflects a complex interplay that can influence multiorgan function and vascular permeability [3]. The systemic inflammatory response following CPB in adults has earlier been described in previous studies. However, the dynamics of cytokine release and their specific roles in the pathophysiology of LCOS in the pediatric population still remains unclear.
Understanding the dynamics of cytokine release following open heart surgery is crucial for optimizing management strategies, minimizing adverse effects, and improving outcomes in pediatric patients. Hence, this study aimed to elucidate the patterns of both proinflammatory and anti-inflammatory cytokine levels (IL-1β, IL-6, IL-8, IL-10, TNF-α, and C-reactive protein [CRP]) in the context of pediatric cardiac surgery with CPB. Additionally, it aimed to evaluate the association of these cytokines with LCOS-related outcomes.
Methods
1. Study design, setting, and participants
This prospective observational cohort study was performed in an 8-bed pediatric intensive care unit (PICU) at Songklanagarind Hospital, Hat Yai, Songkhla; Thailand. Children aged 1 day to 18 years having undergone elective open cardiac surgery with CPB, and admitted to the PICU postoperatively from August 2022-April 2023 were included in this study. Those excluded from this study were as follows: preterm infants (gestational age <37 weeks), patients weighing less than 2 kg, patients that could not be weaned off CPB and required extracorporeal membrane oxygenation (ECMO) before leaving the operating room, and whose parents or legal guardians refused to give informed consent. Information pertaining to the research was explained to parents/guardians at the outpatient clinic and signed informed consent was obtained on the date of admission for preoperation.
This study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Prince of Songkla University (IRB 64-299-1-1: date of approval: August 3, 2021. Study title: Predictability of central venous to arterial carbon dioxide difference and inflammatory markers in children with cardiac surgery to poor outcomes). The study was conducted in accordance with guidelines for Good Clinical Practice and with the 1975 Declaration of Helsinki. Written informed consent forms were obtained preoperatively from either the parents or legal guardians.
2. Surgical procedures
Anesthesia was initiated according to our standardized protocol. Following incision, heparin was administered intravenously at a dosage of 300 units/kg to achieve an activated clotting time (ACT) exceeding 400 seconds. ACT was monitored at 30-minute intervals throughout the procedure, with an additional heparin dose of 300 units/kg provided if the ACT fell below 400 seconds. Intraoperative corticosteroids were administered intravenously, based on individual anesthesiologist preferences. The priming solution for the CPB circuit included either Ringer’s lactate or normal saline with mannitol, along with 20% albumin for patients weighing less than 10 kg. Leukocyte-depleted packed red blood cells were utilized when the preoperative hematocrit was below 30%. CPB was performed using a CAPIOX FX05 Oxygenator and LivaNova Sorin Stockert S5 Heart-Lung Machine, following the α-stat strategy at a target temperature of 28°C. Hypothermia was induced to varying degrees; depending on the surgical procedure. Antegrade cardioplegia was administered at a volume of 20 mL/kg to achieve cardiac arrest, with an additional 10 mL/kg given if aortic cross-clamp time exceeded 20 minutes. The Selection of cardioplegia is based on the anticipated duration and complexity of the surgical procedure. This may compass a conventional solution, a modified del Nido solution, or Custodiol-histidine-tryptophan-ketoglutarate solution. Pump flow rates were maintained between 100–150 mL/kg/min for infants and 2.5–3.0 L/m²/min for older patients: adjusted according to age-appropriate mean arterial pressure. Arteriovenous modified ultrafiltration was selectively applied, based on the consensus of the surgical team and perfusionist, and performed for 5 minutes after separating from CPB. At the conclusion of CPB intravenous protamine sulfate was administered to reverse heparin anticoagulation.
3. Data collection and measurement
Inflammatory markers were analyzed from blood sampling collected from the arterial line at 5 different time points; including preoperative (preop), immediate (T0), 6-hours (T6), 12-hours (T12), and 24-hours (T24), after PICU arrival. The attending staff independently provided standard care; including fluid resuscitation, vasopressor-inotropic adjustment and corticosteroid prescription, for postoperative care; without the researcher’s involvement. Our institute’s protocol for cardiac surgery care, is that corticosteroid is not prescribed, unless patients have suspected septic shock or require inotropic agents of more than two. For post operative states, if CPB is longer than 60 minutes, a single dose of dexamethasone 0.6 mg/kg (maximum 10 mg) would be prescribed. However, if patients develop hemodynamic instability persistently after operation, hydrocortisone 1 mg/kg/dose q 6 hours would be considered by individuals of the attending staff.
The serum samples, which were isolated from blood specimens, were transported to the Unit of Immunology and Virology within an hour, at room temperature. The serum samples were separated by centrifuge at 1,000x g for 15 minutes at 4°C, then aliquoted in a Cryovial tube and kept at -80°C, until used for measuring target cytokines. Five human cytokines (TNF-α, IL-1β, IL-6, IL-8, IL-10) were quantified using Bio-Plex Pro Human Screening Panel 5plex XPLEX, Bio-Rad, USA); following the manufacturer’s instruction. The level of targeted cytokines was determined on a Bio- Plex 200 array reader (Bio- Rad) and analyzed using Bio- Plex Manager software. The serum samples were tested in duplicate, and cytokine levels were quantified via comparison to the standard curve.
Patients’ data included: general baseline characteristics, intraoperative parameters, postoperative interventions and outcomes, which were collected until patients were discharged. Risk Adjustment for Congenital Heart Surgery (RACHS) score; ranging from 1 to 6, was used to classify the severity of cardiac surgery [4]. This was created to determine the risk of hospital mortality after undergoing cardiac surgery: higher numbers represent higher risk of death. Sepsis before operation was determined if the patient received any intravenous antibiotics within 7 days before the operation date. Reintubation was counted if any patient was re-intubated within 72 hours after extubating. Patients requiring either medical or electrical conversion of arrhythmias after PICU arrival were determined as having significant arrhythmias. The vasoactive-inotropic score (VIS) was based on the formula of Gaies et al. [5], as follows: VIS=dopamine dose (μg/kg/min)+dobutamine dose (μg/kg/min)+100×epinephrine dose (μg/kg/min)+10×milrinone dose (μg/kg/min)+100 × norepinephrine dose (μg/kg/min)+10,000×vasopressin dose (U/kg/min). Acute kidney injury was diagnosed according to the Kidney Disease Improving Global Outcomes guidelines; 2012 [6].
The primary endpoint was LCOS-related outcome, which was modified from previous studies [7,8]. These composite outcomes were fulfilled if at least two of the following criteria were met within 24 hours postoperatively: (1) clinical LCOS : prolonged capillary refill >3 seconds, systolic blood pressure <5th percentile for age and gender, persistent elevation of left atrial pressure >10 mmHg for at least 6 hours, low urinary output (<1 mL/kg/hr) for at least 6 hr despite diuretic use; (2) laboratory LCOS: persistently elevated lactate level (>2 mmol/L), metabolic acidosis with an increase in the base deficit (>4 mmol/L) for at least 6 hours consecutively; (3) VIS ≥ 20; (4) cardiac arrest, or utilization of ECMO; and (5) left ventricular fraction <50% on echocardiography on the immediate postoperative day.
4. Statistical analyses
Statistical analyses involved descriptive analysis of means (standard deviations [SDs]) for normally distributed continuous data, medians (interquartile ranges [IQR]) for nonnormally distributed continuous data, and percentages in categorical data. The Student t test or Mann-Whitney U test was used for intergroup comparison of continuous data, depending on the pattern of data distribution. One-way repeated measures analysis of variance test was performed to examine the mean difference of each cytokine in overall participants. Linear mixed-model regression was used to evaluate the differences of cytokines values between the LCOS and non-LCOS groups over time. The delta value of each cytokine was calculated by the value at the first significant time point, which was identified by the linear mixed-model method minus the preoperative value: optimal cut off delta value to predict LCOS was calculated by Youden method. Baseline variables that would affect outcomes; including significant cytokines, were included in the multivariate logistic regression analysis. Multicollinearity was assessed using variance inflation factor. The final model was selected using a stepwise backward elimination method based on the likelihood ratio test and Akaike information criterion. Independent risk factors for LCOS were presented as adjusted odds ratios (ORs) with 95% confidence intervals (CI). Areas under the receiver operating curves were applied to discriminate the model performance in predicting LCOS. All analyses were conducted using R ver. 4.3.1 (The R Foundation for Statistical Computing, Austria).
Results
1. Baseline characteristics of the study patients
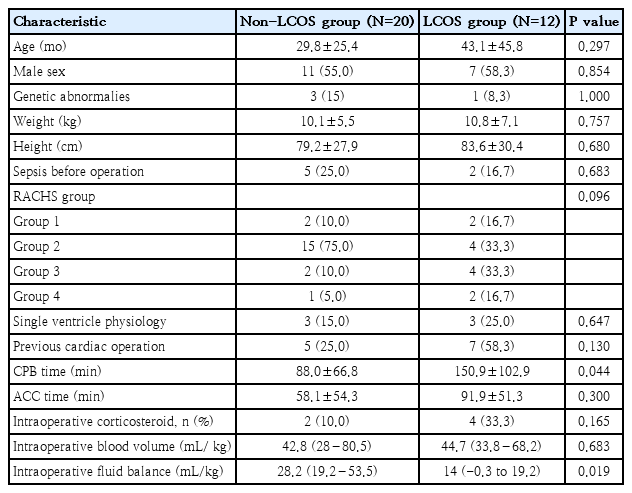
Thirty-six patients were enrolled for cardiac surgery during the study period. Three patients were excluded, due to an emergency operation, and one being unweanable CPB: leaving 32 patients for final analysis. Baseline characteristics and outcomes are summarized in Table 1. The majority of patients were male (56.2%, 18 of 32), with a mean age of 34.8 months (SD, 34.4). Nine patients (28.3%) had RACHS category ≥ 3, 12 (37.5%) had had previous cardiac surgery. Type of cardiac surgery is shown in Supplementary Table 1. The LCOS-related outcome accounted for 37.5% (12 of 32), and the hospital mortality rate was 3.1% (1 of 32). Patients who experienced LCOS-related outcomes had significantly longer duration of CPB time and lower intraoperative fluid balance than those who did not, whereas age, sex and preoperative status showed no significant differences (Table 2).
2. Cytokines and association with outcomes
All cytokines’ values, except IL-1β, increased significantly post operation compared with preoperative state. IL-6, IL-8, IL-10, and TNF-α had peak values at the time of PICU arrival, which declined overtime, while CRP levels rose slower; reaching peak level at 24- hours after operation (Supplementary Fig. 1).
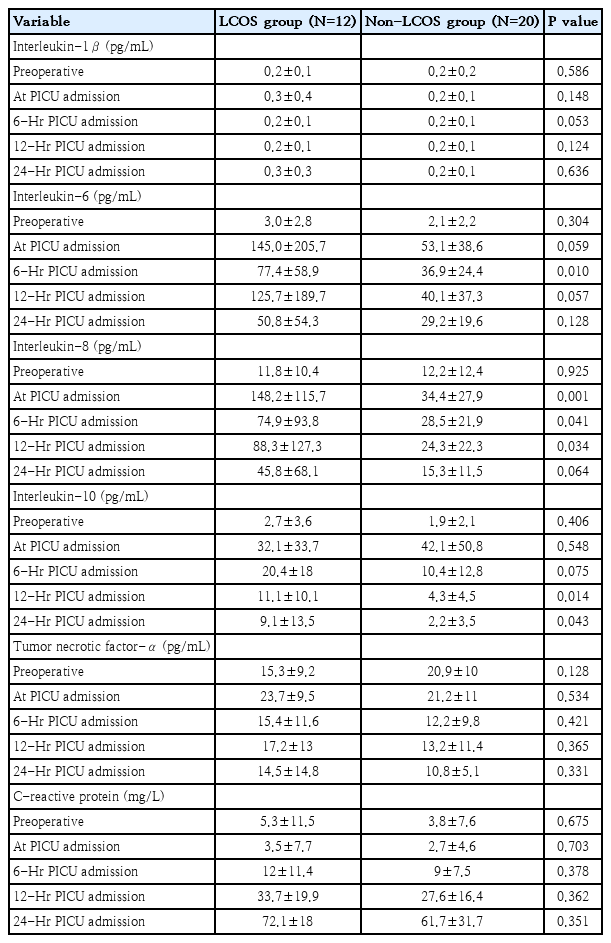
Comparing cytokine values between patients with and without LCOS-related outcomes at each time point by univariable analysis: all markers were not significantly different at preoperation. IL-6, IL-8, IL-10 revealed significantly higher values in LCOS compared with non-LCOS groups at different time points, while IL-1β, TNF-α and CRP between the 2 groups were not different. The significant higher values in the LCOS group were detected at T6 in IL-6 (77.4±58.9 vs. 36.9±24.4, P=0.010), at T0, T6 and T12 in IL-8 (148.2±115.7 vs. 34.4±27.9, P=0.001; 74.9±93.8 vs. 28.5±21.9, P=0.041; 88.3±127.3 vs. 24.3±22.3, P=0.034 in T0, T6, and T12, respectively) and at T12 and T24 in IL-10 (11.1+10.1 vs. 4.3+4.5, P=0.014; 9.1+13.5 vs.2.2+3.5, P=0.043 in T12 and T24 respectively) (Table 3).
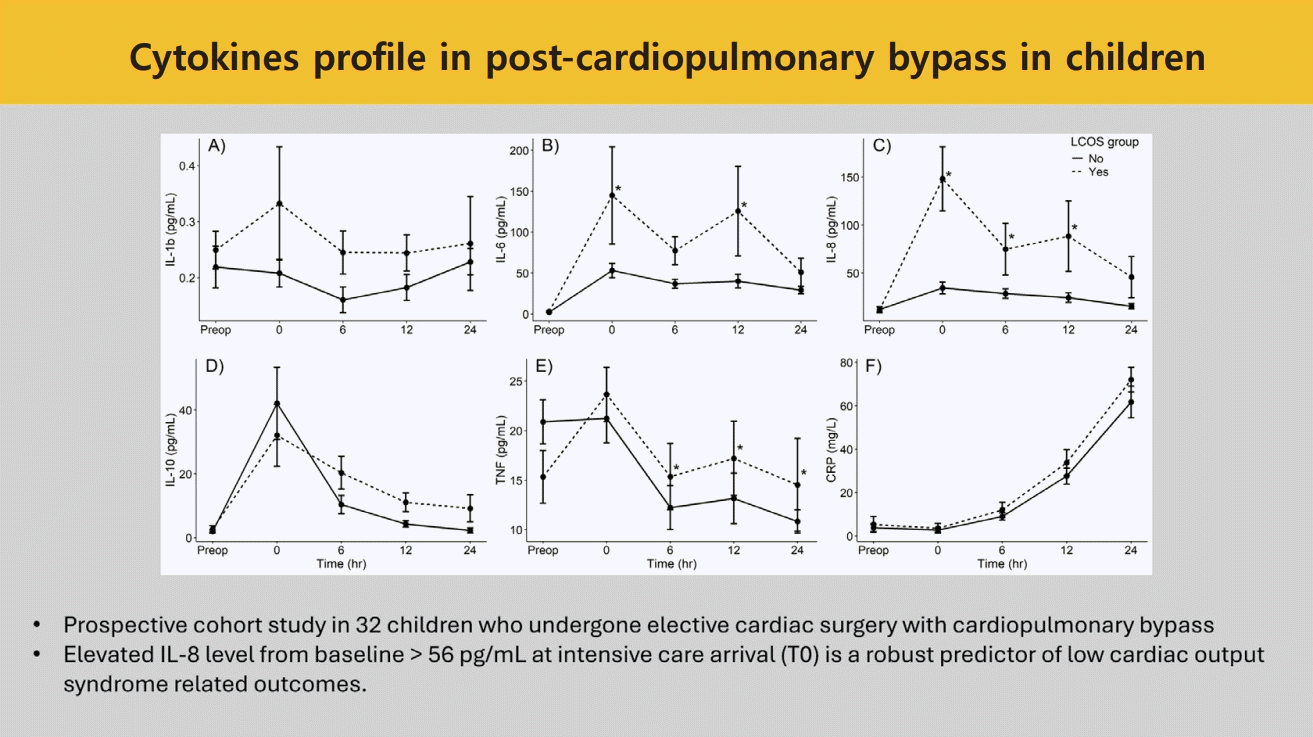
However, after performing linear mixed-model regression analysis, the IL-6, IL-8 and TNF-α between the LCOS and non-LCOS groups were significantly different: as shown in Fig. 1. The first significant time point of IL-6 and IL-8 were at the time of PICU arrival, in contrast TNF-α started to be revealed at significant levels 6-hours after PICU admission. There were no significant different values between the 2 groups in IL- 1β, IL-10, and CRP.
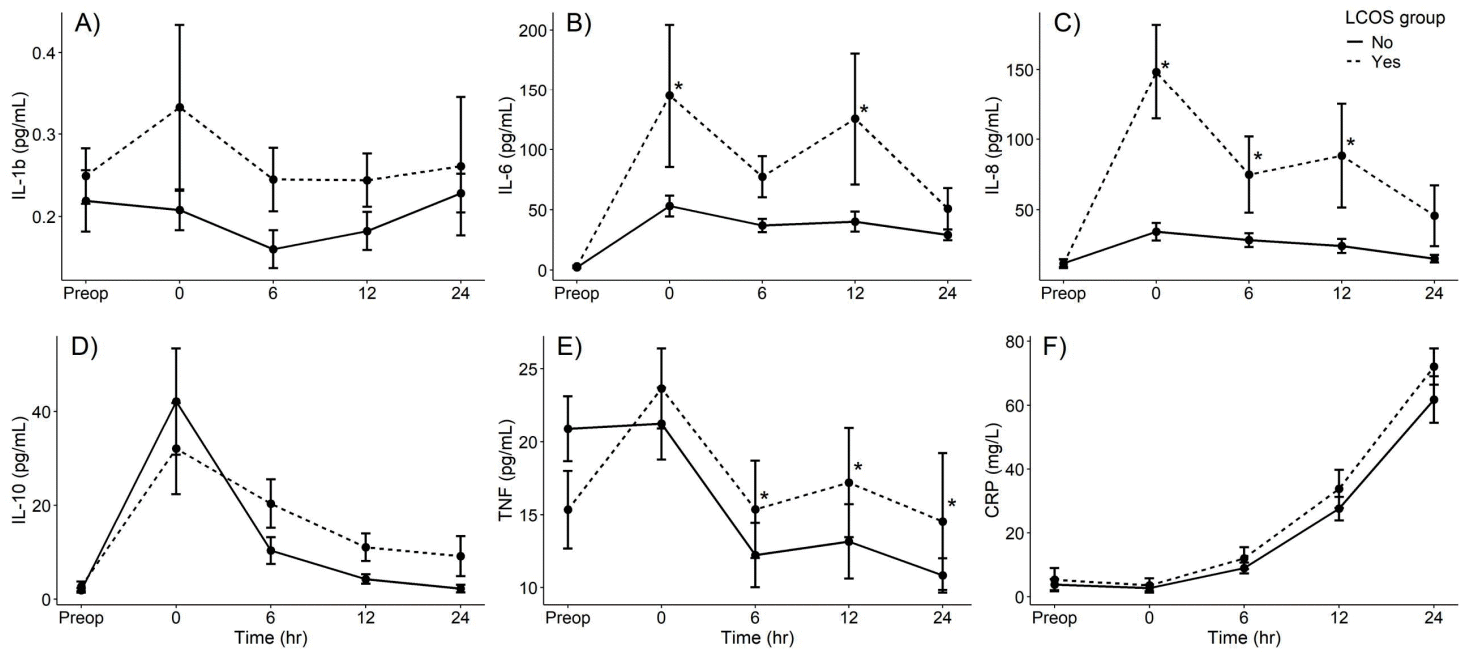
Cytokine values preoperatively and at 0-, 6-, 12-, and 24-hour post–pediatric intensive care unit admission of patients with versus without LCOS-related poor outcomes: (A) interleukin (IL)-1β, (B) IL-6, (C) IL-8, (D) IL-10, (E) tumor necrotic factor (TNF)-α, (F) C-reactive protein (CRP). The dashed line represents patients with LCOS-related outcomes, while the solid line represents patients without LCOS-related outcomes. *P<0.05 on a linear mixed-model regression analysis comparing the differences in cytokine values between the LCOS and non-LCOS groups over time. LCOS, low cardiac output syndrome.
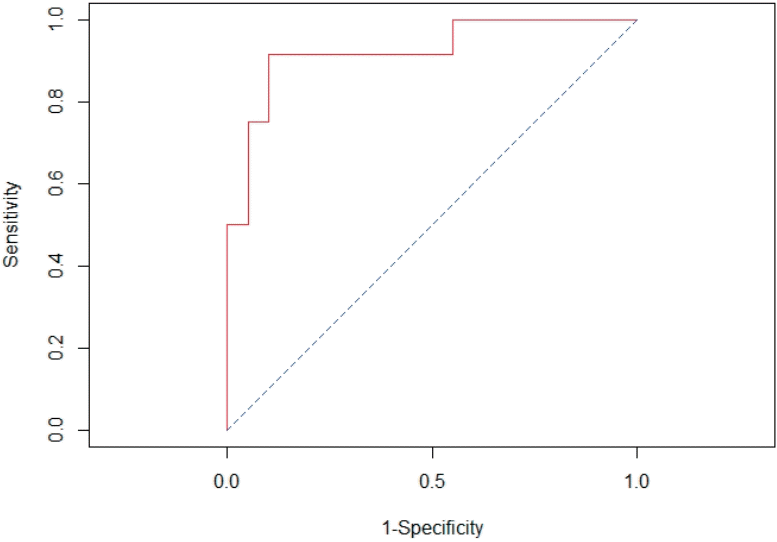
The earliest time that showed significance of each cytokine (T0 in IL-6 and IL-8 and T6 in TNF-α) was selected to calculate the delta value. The best cut off delta values identified by Youden method, were as follows: >90 pg/mL for IL-6, >56 pg/mL for IL-8 and >0 pg/ml for TNF-α. The final model of multivariable logistic regression analysis revealed that increasing levels of IL-8 at time of PICU admission of more than 56 pg/mL from preoperative and intraoperative corticosteroid administration had significant association with LCOS-related outcomes (Table 4). The AUC of this model was 0.92 (95% CI, 0.82–1.00) (Fig. 2).
Discussion
Although patient characteristics did not differ significantly between the LCOS and non-LCOS groups, patients in the LCOS group were more likely to have undergone complex cardiac surgeries, as indicated by higher RACHS scores, single ventricle physiology, and a history of previous cardiac operations. Our study reported a significant increase in all markers, except IL-1β, after bypass was performed in children undergoing open cardiac surgery. CRP was identified as a late-response marker compared to others. Significant differences were observed in IL-6, IL-8, and TNF-α levels between patients that developed LCOS and those whom did not. Notably, an increase in IL-8 > 56 pg/mL from baseline postoperation demonstrated the most significant effect on LCOS-related outcomes.
The pattern of both proinflammatory and anti-inflammatory cytokine changes within 24 hours postsurgery in this study were consistent with previous findings [9-12]. The nonsignificant change in IL-1β and TNF-α postoperation may be attributed to their rapid production and clearance during surgery; as described by Misoph and Babin-Ebell [13]. Additionally, the late release of CRP aligns with observations in the study by Chew et al. [12]. The most commonly reported cytokines associated with clinical outcomes in children undergoing CPB were: IL-6, IL-8, and IL-10. Carmona et al. [8] measured inflammatory mediators (IL-6, IL-8, IL-10, and TNF-α) in 46 pediatric cardiac patients, and found that participants that developed LCOS had higher levels of IL-6, IL-8, and IL-10 at various postoperative time points; although, no significant differences were observed in TNF-α levels. Multivariate logistic regression identified postoperative IL-8 at 4 hours ≥128 pg/mL as being significantly associated with LCOS events. A more recent study in 31 patients with congenital heart disease found that higher preoperative IL-10 and 24-hour postoperative IL-8 levels were significantly linked to LCOS [7]. These findings align with our study, which identified IL-8 as having the most significant impact on LCOS-related outcomes. While initial t test analysis indicated differences in IL-6, IL-8, and IL-10 levels between patients with and without LCOS, linear mixed-model regression revealed that IL-6, IL-8, and TNF-α were significantly associated with LCOS outcomes; whereas, IL-1β, IL-10, and CRP were not. Furthermore, changes in IL-8 between pre- and immediate postoperation significantly influenced primary outcomes. Several studies have highlighted a correlation between IL-8 and CPB duration, while findings for IL-6 and TNF-α remain inconclusive [3,14-16]. IL-8 plays a pivotal role in neutrophil chemotaxis, inducing vascular leakage, which may contribute to inadequate cardiovascular perfusion [9].
The nonsignificance of preoperative markers in predicting LCOS outcomes in this study may be due to the elective surgery setting; wherein, patients typically had stable hemodynamics preoperatively. In contrast, significant preoperative IL-10 and N-terminal pro-B-type natriuretic peptide levels in previous studies might reflect unstable hemodynamics.
Other clinical outcomes; such as postoperative treatment and intensive care unit (ICU) length of stay, were not addressed in this study due to its small sample size. However, studies have found correlations between higher IL-8 levels and increased inotropic support, blood transfusion requirements, longer ventilation and ICU stays [9,15,17,18]. Similarly, elevated postoperative IL-6 and IL-10 levels have been associated with extended inotropic use, intraoperative blood transfusion, prolonged ventilation and ICU stays [9,11,19-21].
The strength of this study lies in the inclusion of a varied age range as well as different types of congenital heart disease. It also evaluated dynamic cytokine changes over time and explored optimal cutoff points for predicting LCOS. However, a key limitation was the lack of a definitive gold standard for LCOS diagnosis. We modified previously published definitions to incorporate both clinical and laboratory conditions for a more accurate diagnosis. Additionally, intraoperative evaluations were omitted, as we focused on the postoperative period when patients' clinical conditions are typically more unstable than during surgery. Lastly, this study was limited in elective surgery participants with stable hemodynamic status. Further research on different populations; such as emergency operations in those having unstable conditions, would show different results.
In conclusion, the increase of IL-8 of more than 56 pg/mL is significantly associated with LCOS-related poor outcomes. This may well be the next step of parameters to evaluate LCOS-related outcomes. Understanding these dynamics is essential for developing targeted interventions to both improve recovery and reduce complications in pediatric cardiac surgery.
Supplementary materials
Supplementary Table 1 and Supplementary Fig. 1 are available at https://doi.org/10.3345/cep.2025.00836.
Details of heart disease and type of surgical procedures
Cytokines values at preoperation, at intensive care unit admission, 6-, 12-, and 24-hour postoperation via repeated 1-way analysis of variance test. IL, interleukin; TNF, tumor necrosis factor; CRP, C-reactive protein. *P<0.05. **P<0.01. ***P<0.001. ****P<0.0001.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was financially supported by the Faculty of Medicine, Prince of Songkla University (grant number: 64-087-2).
Acknowledgments
We would like to thank the Translational Medicine Research Center (TMRC), Faculty of Medicine, Prince of Songkla University, for assistance with sample collection and analysis and also thank Jirawan Jayuphan, Statistician, Department of Epidemiology, Faculty of Medicine, Prince of Songkla University, for assistance with data analysis.
Author contribution
Conceptualization: PP, JJ, SS; Formal Analysis: PP; Investigation: PP, KS, JJ, SS; Methodology: PP; Project Administration: PP; Writing-Original Draft: PP; Writing -Review & Editing: KS, JJ, PD, SS, KR