Ciclesonide shows a lung-protective effect in neonatal hyperoxia-exposed rats
Article information
Abstract
Background
Bronchopulmonary dysplasia (BPD), a chronic lung disease primarily observed in premature infants, is attributed to a lung injury–repair imbalance. Studies of postnatal corticosteroids have failed to identify clear candidates to help alleviate high BPD rates without risks or adverse effects.
Purpose
This study aimed to assess whether the systemic postnatal administration of an alternative glucocorticoid, ciclesonide, could attenuate alterations in lung structure and right ventricular hypertrophy in a hyperoxic rat BPD-like model.
Methods
In a hyperoxia-induced model of BPD-like lung injury, pups were maintained in oxygen-enriched atmosphere-hyperoxia or normoxia (room air) for 14 days after natural birth, and subcutaneous ciclesonide (0.5 mg/kg) was administered postnatally for 5 consecutive days. On postnatal day 14, lung function (peak inspiratory pressure and compliance), lung structure (radial alveolar count, mean linear intercept, and pulmonary vessel density), and right ventricular hypertrophy were assessed.
Results
On day 14, the effects of hyperoxia exposure were more evident in untreated rats (impaired lung compliance and structure and right ventricular hypertrophy) than in normoxia-exposed animals. Ciclesonide administration was associated with smaller body weight changes and significantly improved lung compliance, alveolarization, lung vascular growth, and right ventricular hypertrophy.
Conclusion
Postnatal ciclesonide administration preserved lung function and structure and prevented right ventricular hypertrophy in a hyperoxic BPD-like model. These findings suggest that postnatal ciclesonide may be an alternative to existing corticosteroids for the treatment of BPD. However, long-term studies are required to validate these findings.
Key message
Question: Bronchopulmonary dysplasia (BPD) is the most prevalent chronic lung disease of prematurity. Numerous nonpharmacological/pharmacological interventions have been investigated without clear consensus. Can ciclesonide, a new synthetic glucocorticoid, effectively treat BPD?
Finding: Ciclesonide mitigated hyperoxia-induced lung injury and right ventricular hypertrophy in newborn rats.
Meaning: These findings suggest that postnatal ciclesonide may be an alternative to existing corticosteroids for the treatment of BPD.
Introduction
Bronchopulmonary dysplasia (BPD) is the most prevalent chronic lung disease of prematurity, preterm infants born earlier than 28 weeks of gestation having a significant risk of developing this pathology. The injury to immature lungs may occur during antenatal and/or early postnatal life (due to infection, hyperoxia, or mechanical ventilation, among other factors). BPD and prematurity have an elevated cost for families and healthcare systems [1], due to an increased risk of mortality, long-term respiratory and cardiovascular impairments, growth failure, and neurodevelopmental delays [2]. Numerous nonpharmacological and pharmacological interventions have been investigated seeking to prevent and manage BPD; however, there is still no clear consensus on the best medication for BPD treatment. Since the incidence of BPD can be expected to increase in the coming years, as the incidence of preterm birth and survival rates of preterm infants keep rising, there is an urgent need to find new therapies.
Among other medications, the use of postnatal corticosteroids (potent anti-inflammatory drugs) for BPD treatment [3-6] has been widely evaluated in preclinical and clinical trials. Multiple systemic (mainly dexamethasone, betamethasone, hydrocortisone), inhaled or intratracheal (mainly budesonide) corticoids [7-9] at different doses, at different postnatal ages, and for different durations have been tested. Nonetheless, no clear candidate has emerged to treat BPD without a risk well-documented adverse effects (intestinal perforation, growth failure, arterial hypertension and negative impacts on the developing brain) [5,10]. Ciclesonide is a new generation inhaled synthetic glucocorticoid currently approved for the treatment of asthma and allergic rhinitis. It is a prodrug that is converted by carboxylesterases into the active compound desciclesonide in the lung. Given its characteristics, it is a potential candidate to prevent or treat BPD, that can also be administered systematically and has shown both selective activation of the glucocorticoid receptor in the neonatal lung, and limited adverse neurodevelopmental effects in a healthy neonatal rat model [11,12]. Moreover, ciclesonide has shown a positive effect (preserved lung function and structure with prevention of right ventricular hypertrophy) in a BPD-like model based on antenatal intraamniotic enterotoxin administration seeking to produce intrauterine inflammation, as observed in chorioamnionitis [13].
The pathogenesis of BPD is complex and multifactorial, involving antenatal and/or postnatal factors (such as prematurity, chorioamnionitis, mechanical ventilation, and hyperoxia), and hence, it is essential to find therapeutic strategies that address a wide range of factors involved in the development of BPD. Taking into account the positive effect of ciclesonide observed in our previous study with a model of BPD based on antenatal enterotoxin exposure, we hypothesize that ciclesonide would reduce the alterations associated with long exposure to oxygen (hyperoxia) in the neonatal lung.
Methods
The experimental protocol meets European and Spanish regulations for the protection of experimental animals (UE2010/63 and RD53/2013) and has been approved by the Ethics Committee for Animal Welfare of the Biobizkaia Health Research Institute (OEBA-CET-2021-002).
1. Study design
1) Hyperoxic model
Timed-pregnant Sprague Dawley rats were housed individually in standard cages with food and water ad libitum under a 12 hours light cycle and allowed to give birth. The pups were pooled and randomized within 12 hours of birth and returned to the dams. Newborn rats were randomly assigned to normoxia (room air, RA) or oxygen-enriched atmosphere-hyperoxia (HYP). The pups in HYP groups were reared with the dams in an atmosphere containing 80-85% oxygen (Proox Model 360, BioSpheric, USA) from postnatal days 0 (PN0) to 14 (PN14); in parallel, the pups in the RA group were reared with the dams under normoxic conditions. To avoid oxygen toxicity, the nursing mothers were rotated between oxygen treatment and RA litters every 24 hours.
2) Groups
- Room air group (RA, n=10): Neonatal rats were maintained in 21% oxygen and received a subcutaneous (s.c.) injection of normal saline, the same as that used as a vehicle for drug administration in other groups.
- Room air plus ciclesonide group (RA-Cicle, n=8): Neonatal rats were maintained in 21% oxygen and received an s.c. injection of ciclesonide (0.5 mg/kg, SML1955, Sigma-Aldrich, USA) at the nape of the neck (10 μL/g) once a day for 5 consecutive days (PN1–PN5) [11,12].
- Hyperoxia group (HYP, n=10): Neonatal rats were maintained in 80-85% oxygen and received an s.c. injection of normal saline.
- Hyperoxia plus ciclesonide group (HYP-Cicle, n=12): Neonatal rats were maintained in 80%–85% oxygen and received an s.c. injection of ciclesonide (0.5 mg/kg, SML1955, Sigma-Aldrich) at the nape of the neck (10 μL/g) once a day for 5 consecutive days (PN1–PN5) [11,12].
2. Study measurements
1) Body weight and survival rate
Survival rate was recorded at birth and on PN14. Body weight was measured within 30 minutes after birth, at PN7, and just before pups were sacrificed for study measurements.
2) Lung mechanics
On PN14, neonatal pups were anesthetized, and a catheter was placed in their trachea and connected to a small animal ventilator (VentElite, Harvard Apparatus, USA) with the following settings: tidal volume of 6 mL/kg, positive end-expiratory pressure of 3 cmH2O, and a respiratory rate of 90 bpm. The peak inspiratory pressure (PIP) reached by each neonatal pup under the preset ventilation settings was measured and lung compliance was calculated: tidal volume/[(PIP-positive end-expiratory pressure)*body weight] [13].
3) Lung tissue processing and analysis
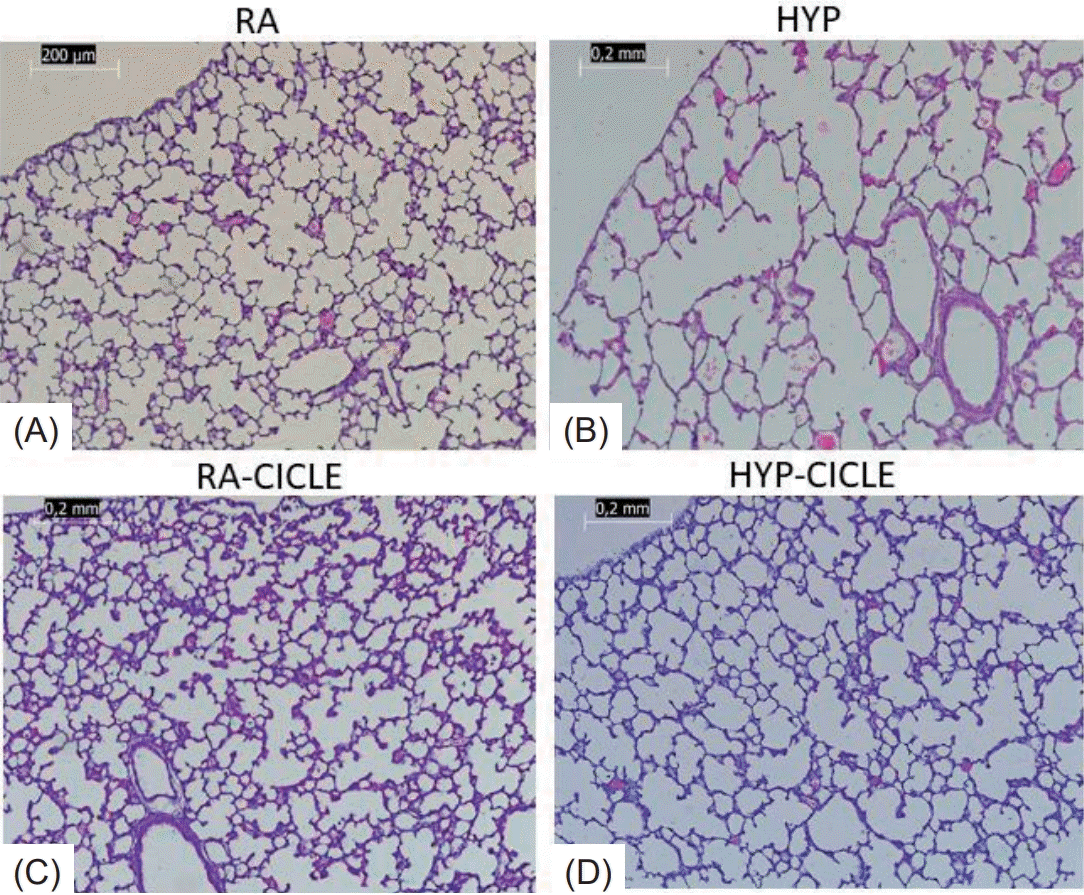
After lung mechanics measurements, the pups were sacrificed using pentobarbital. For fixation, the lungs were inflated with 4% formaldehyde administered through a Luer lock cannula inserted into the throat and maintained at 15 cmH2O pressure for 60 minutes. A ligature was tightened around the trachea to maintain the pressure, and the cannula was removed. The lungs were then dissected and immersed in 4% formaldehyde at room temperature overnight. Subsequently, 2-mm thick transverse slices were cut from lung samples and embedded in paraffin. Pathologists blinded to group allocation conducted the following lung immunohistochemical and morphometric analyses.
Five micrometer sections were taken, mounted on slides and stained with hematoxylin and eosin for the assessment of alveolar structure by morphometric analysis (radial alveolar count [RAC], and mean linear intercept [MLI]); and with von Willebrand Factor (vWF), an endothelial cell-specific marker, for the assessment of pulmonary vessel density (PVD) and pulmonary vessel wall thickness (PVWT). In each case, we obtained at least 5 readings for RAC and measured at least 10 pulmonary vessels for PVD and PVWT.
RAC: Alveolarization was assessed using standard RAC methods developed by Emery and Mithal [14,15]. Specifically, respiratory bronchioles were identified as bronchioles lined by epithelium in one part of the wall, and we counted the number of septae intersected by a perpendicular line drawn from the center of the respiratory bronchiole to the edge of the acinus connective tissues, septum, or pleura.
MLI: we superimposed a grid on the image, counted the number of times the alveolar walls were intercepted by the grid lines, and then used the formula, MLI = (N)(L)/m, where N is the number of superimposed lines, L the length of the superimposed lines, and m the number of times the alveolar walls are intercepted by the grid lines.
PVD: we counted the number of vWF-stained vessels with an external diameter of less than 100 μm per high-power field, excluding fields containing large airways or vessels from the analysis.
PVWT: we measured wall thickness and external diameter in pulmonary vessels with an external diameter of 10–30 μm using Adobe Photoshop CS, and calculated the percent medial thickness of an individual vessel using the following formula: (medial thickness×2×100)/external diameter.
4) Right ventricular hypertrophy measurements
The heart was isolated and the right ventricle (RV) and left ventricle plus septum (LV+S) were dissected and weighed. We then calculated the RV-to-LV+S weight ratio, to assess right ventricular hypertrophy.
3. Data analysis
Values were expressed as mean±standard deviation. Intragroup comparisons were made using a one-factor analysis of variance (JMP8, Statistical Discovery, USA). Analyses were performed with the Bonferroni-Dunn correction. P values <0.05 were considered significant.
Results
1. Body weight and survival rate
At PN14, all neonatal rats kept in 21% oxygen (RA and RA-Cicle groups) were alive. In contrast, the survival rate was just 40% in the HYP group. Notably, however, when ciclesonide was administered during hyperoxia exposure, the survival rate was markedly higher 80% at PN14 (the HYP-Cicle group).
At birth, all the pups showed similar body weights. At PN7, no differences were observed in body weight between the untreated groups (RA and HYP), while at PN14, the HYP group had a higher body weight than the RA group. Pups administered ciclesonide and maintained in room air (the RA-Cicle group) had significantly lower body weights than those in the RA group at both PN7 and PN14 (Fig. 1). A similar effect was observed when ciclesonide was administered during hyperoxia, body weight being significantly lower in the HYP-Cicle than the HYP group (Fig. 1).
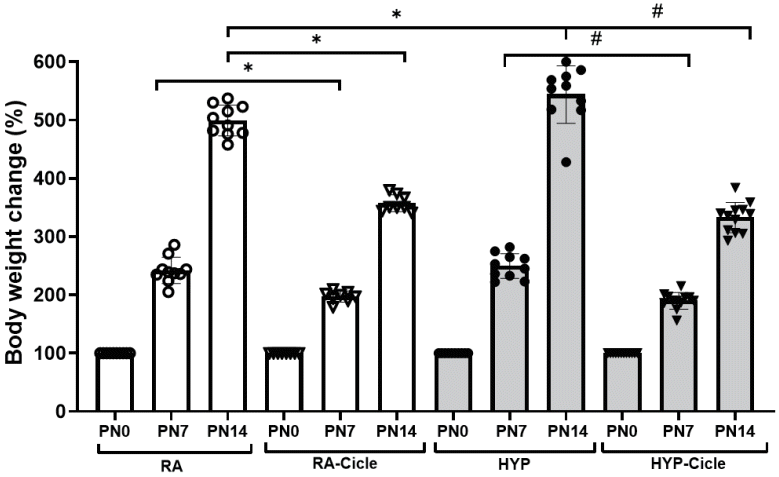
Effect of ciclesonide administration on body weight change in an experimental hyperoxia model. Values of body weight change (%) in newborn pups assigned to normoxia (room air [RA]), oxygen-enriched atmosphere-hyperoxia (HYP), or subcutaneous administration of ciclesonide during normoxia (RA-Cicle) or after hyperoxia (HYP-Cicle). PN0, postnatal day 0; PN7, postnatal day 7; PN14, postnatal day 14. Number of pups studied in each group: RA, n=10; RA-Cicle, n=8; HYP, n=10; HYP-Cicle, n=12. *P<0.05 vs. RA group. #P<0.05 vs. HYP group.
2. Lung mechanics
Comparing the untreated pups, at PN14, significantly higher PIP with significantly lower compliance values were observed in the HYP group than in the RA group at PN14 (Fig. 2A and B). Meanwhile, PIP and compliance in pups exposed to subcutaneous ciclesonide treatment (the RA-Cicle and HYP-Cicle groups) were better than in the RA and HYP groups.
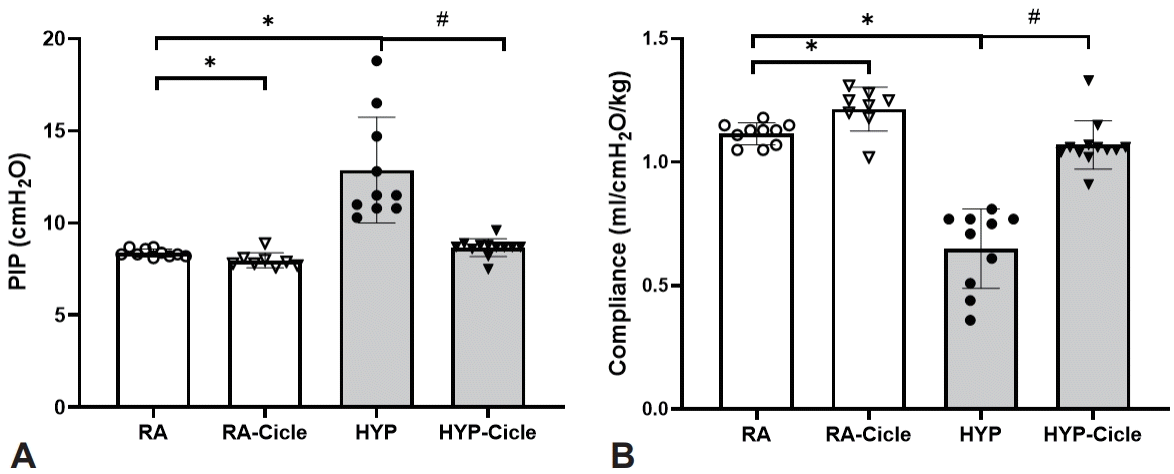
Effect of ciclesonide administration on lung function in experimental hyperoxia model. Values of peak inspiratory pressure (PIP) (A) and lung compliance (B) in newborn pups assigned to normoxia (room air [RA]), oxygen-enriched atmosphere-hyperoxia (HYP), or subcutaneous administration of ciclesonide during normoxia (RA-Cicle) or after hyperoxia (HYP-Cicle). Number of pups studied in each group: RA, n=10; RA-Cicle, n=8; HYP, n=10; and HYP-Cicle, n=12. *P<0.05 vs. RA group; #P<0.05 vs. HYP group.
3. Lung histological morphometric analysis
Comparing the untreated groups, at PN14, the HYP group showed less alveolarization (RAC) (Fig. 3A) and lower PVD (Fig. 3B) together with higher MLI values (Fig. 3C) than the RA group. Regarding the impact of ciclesonide treatment, the HYP-Cicle group had better RAC (Fig. 3A) and MLI (Fig. 3C) than the HYP group (Fig. 4). Further, the RA-Cicle group showed lower PVWT values than the RA group, with no differences being observed between other groups (Fig. 3D).
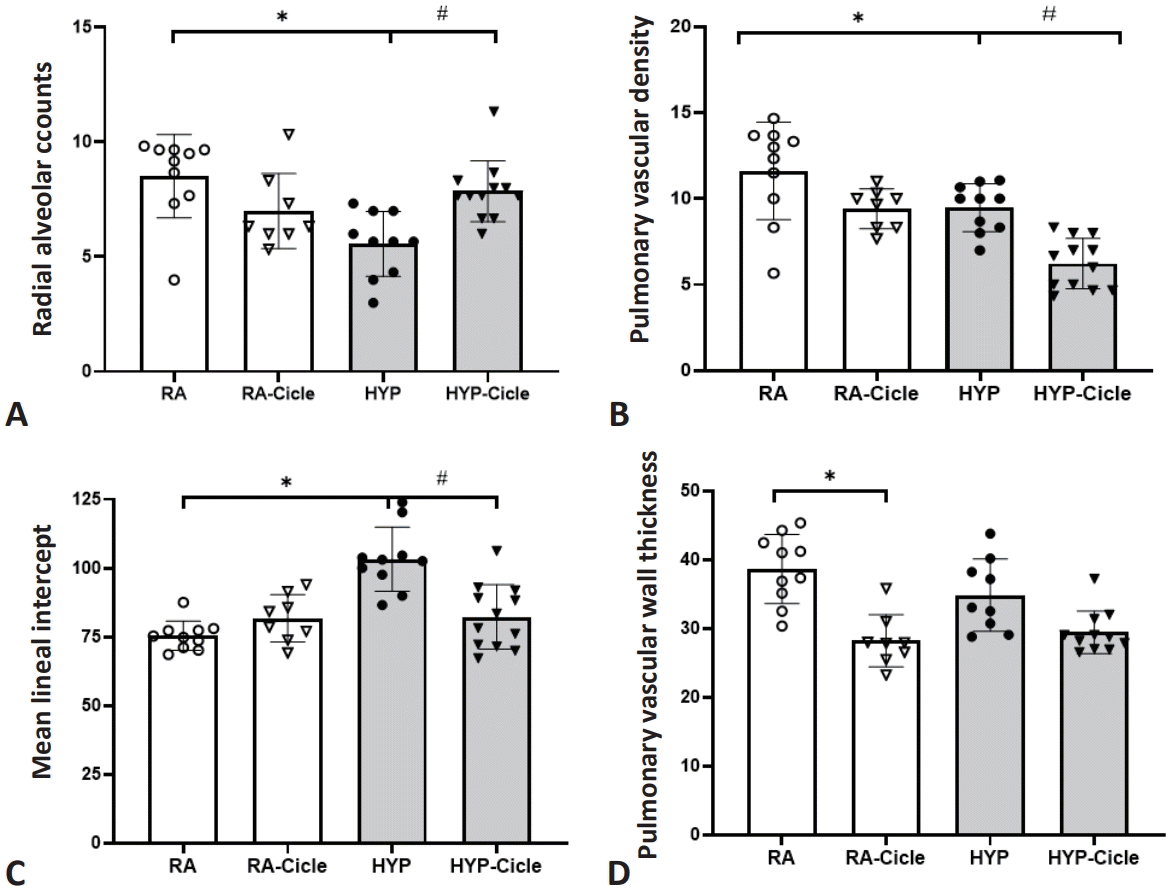
Effect of ciclesonide administration on lung morphometry in experimental hyperoxia model. Radial alveolar counts (A), pulmonary vascular density (B), mean lineal intercept (C), pulmonary vascular wall thickness (D) in newborn pups assigned to normoxia (room air [RA]), oxygen-enriched atmospherehyperoxia (HYP), or subcutaneous administration of ciclesonide during normoxia (RA-Cicle) or after hyperoxia (HYP-Cicle). Number of pups studied in each group: RA, n=10; RA-Cicle, n=8; HYP, n=10; and HYP-Cicle, n=12. *P<0.05 vs. RA group. #P<0.05 vs. HYP group.
4. Right ventricular hypertrophy
Considering the untreated groups, right ventricular hypertrophy was observed in neonatal rats exposed to hyperoxia (the HYP group) compared to the RA group at PN14 (Fig. 5). Notably, the neonatal rats exposed to hyperoxia that received ciclesonide treatment (the HYP-Cicle group) showed significantly less right ventricular hypertrophy than the HYP group at PN14 (Fig. 5).
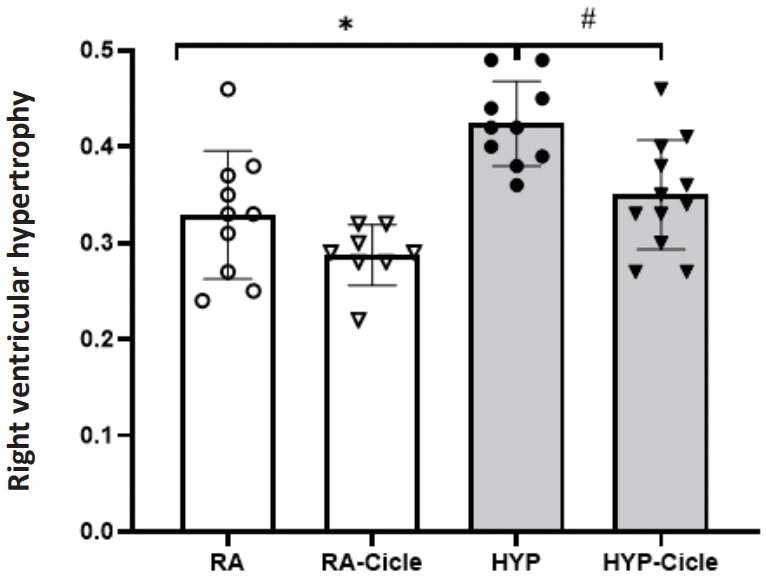
Effect of ciclesonide administration on the heart in experimental hyperoxia model. Right ventricular hypertrophy in newborn pups assigned to normoxia (room air [RA]), oxygen-enriched atmosphere-hyperoxia (HYP), or subcutaneous administration of ciclesonide during normoxia (RA-Cicle) or after hyperoxia (HYP-Cicle). Number of pups studied in each group: RA, n=10; RA-Cicle, n=8; HYP, n=10; and HYP-Cicle, n=12. *P<0.05 vs. RA group. #P<0.05 vs. HYP group.
Discussion
In our study, postnatal exposure of neonatal rats to an oxygen-enriched atmosphere for 14 days was associated with right ventricular hypertrophy and impairment of lung development causing alveolar remodeling characterized by reduced alveolarization and vessel density, together with higher MLI. Ciclesonide, a glucocorticoid little explored in preterm neonates, administered for 5 days seemed to be able to mitigate the effects of hyperoxia on the lung and heart, without affecting lung structure, in newborn rats not exposed to hyperoxia.
The etiology of BPD is multifactorial, and seeking to mimic human BPD, newborn animal lungs may be subjected to various stimuli such as prolonged mechanical ventilation, infection/inflammation, or hyperoxia [16]. Among these, postnatal exposure of neonatal rats to hyperoxia has been extensively used to evaluate treatments in a well-established BPD model [17-20]. In our study, neonatal rats maintained in an oxygen-enriched atmosphere (80%–85% oxygen, i.e., hyperoxia) for 14 days developed significant impairment of lung mechanics compared to neonatal rats maintained in RA (21% oxygen). Moreover, the HYP group also showed altered lung structure (sustained abnormalities of alveolar and vascular growth) combined with signs of pulmonary hypertension (greater right ventricular hypertrophy) [21], which seems to mimic features of human BPD [22].
Glucocorticoids, mainly dexamethasone and hydrocortisone, are frequently used to prevent, treat, or mitigate BPD, due to their anti-inflammatory properties [23]. Nonetheless, the use of these 2 glucocorticoids in preterm infants has been related to short- and long-term adverse effects (gastrointestinal bleeding, poor growth, neurodevelopmental impairment, and adrenal insufficiency, among others) [5,10]. On the other hand, budesonide has strong local anti-inflammatory effects and fewer systemic adverse reactions than other glucocorticoids [24]. It has also shown a positive effect, reducing the incidence of BPD, when administrated by inhalation [6] and no effect on mortality or BPD incidence rates when administered intratracheally in combination with surfactant [9].
Ciclesonide is a synthetic glucocorticoid approved for inhalation treatment of asthma and allergic rhinitis in children and adults that has few systemic side effects [25]. It has been previously reported that systematic delivery of this glucocorticoid prodrug (ciclesonide) in a neonatal healthy rat model activated glucocorticoid receptor transcriptional responses in the lungs, but did not trigger multiple adverse effects caused by dexamethasone [11], making it an ideal candidate for avoiding the systemic and neurologic effects observed when other glucocorticoids are used.
In the development of BPD, pre- and/or postnatal factors (infection/inflammation, mechanical ventilation, and long-term supraphysiologic oxygen exposure) may be involved, and hence, it is important to find a drug that is effective when several factors may be responsible for the development of BPD. In our previous study [13], ciclesonide was successfully used to treat neonatal rats exposed to intra-amniotic enterotoxin [13], but this drug has never been tested in a model where postnatal factors are responsible for the BPD development. In this new study, the postnatal administration of ciclesonide for 5 days to neonatal rats exposed to hyperoxia was associated with significant improvement in lung function (PIP and compliance values) and structure (RAC and MLI), and prevention of right ventricular hypertrophy compared to the group only exposed to hyperoxia (HYP). When ciclesoide was administered to pups kept in RA, improvements were observed in compliance without significant changes in lung structure. The effects of systemic administration of ciclesonide on the lung and heart observed in our model could be explained by the drug’s optimal properties. In particular it is capable of rapidly activating many glucocorticoid responsive genes in neonatal lung [11], and also shows high protein binding in the systemic circulation, high first-pass metabolism in the liver, and short halflife [26], resulting in circulating free corticosteroid levels that potentially lead to fewer systemic adverse effects than those seen with dexamethasone (skin and fur abnormalities, and impaired systemic and brain growth and white matter development) [11]. In contrast to our previous study using intra-amniotic enterotoxin to develop a BPD model [13] and in the research of Jaumotte et al. [11] using healthy newborn rats, in our current study, weight gain reduction was noted in the groups treated with ciclesonide (healthy and lung-injured groups). Such an effect has been reported previously [27] describing a reduction in body weight gain when ciclesonide was administered to healthy adult rats. Moreover, a recent systematic review summarizing the current preclinical literature regarding newborn rodents concluded that postnatal systemic corticosteroid administration may have a negative effect on body weight when administrated to healthy or lung-injured newborn animals (hyperoxia model) [28]. BPD is a multifactorial pathology that is influenced by a variety of prenatal and/or postnatal factors, and hence, it is possible that the magnitude of the positive effect of ciclesonide could be related to the main factor that has induced the lung damage.
Some limitations to the study should be recognized. Firstly, we have evaluated the effect of ciclesonide in a hyperoxic model, not in a multi-hit animal model, which might mimic the clinical situation more closely. Secondly, other anti-inflammatory drugs to determine the mechanisms of ciclesonide related to inflammation were not tested. Finally, this study did not assess systemic inflammatory markers vs. inflammatory markers in the lungs, or other organs evaluation.
In conclusion, our results confirm that the administration of ciclesonide, a new synthetic glucocorticoid, is capable of mitigating the lung injury and ventricular hypertrophy induced by prolonged exposure to oxygen in newborn rats, showing significant improvements in lung mechanics and structure. The results of this study support the view that clinical trials are warranted, and at the time of submitting this paper for publication, the first clinical trial to test the safety and toxicity of inhaled ciclesonide in preterm infants at risk of developing BPD has been registered (NCT06589245).
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported in part through grant PI22/00466 (co-funded by the European Regional Development Fund; “A way to make Europe”) by Instituto de Salud Carlos III. The funder had no role in the design, data collection, data analysis, and reporting of this study.
Author contribution
Conceptualization: CRS, VM, LO, BL; Formal Analysis: CRS; Data Curation: CRS, VM; Methodology: CRS, VM, BL, MAGS; Project Administration: CRS; Funding adquisition: CRS, MAGS; Writing–Original Draft: CRS; Writing – Review & Editing: CRS, BL, LO, MAGS, VM