Less invasive surfactant administration versus intubation-surfactant-extubation: a single-center retrospective study
Article information
Abstract
Background
In recent years, minimally invasive methods have been increasingly utilized for surfactant administration in spontaneously breathing preterm infants with respiratory distress syndrome (RDS) managed with nasal continuous positive airway pressure owing to their feasibility and association with improved respiratory outcomes. However, data are limited from developing countries on the use and effectiveness of these techniques.
Purpose
The primary objective of this study was to evaluate the effect of less invasive surfactant administration (LISA) and intubation-surfactant-extubation (InSurE) techniques on the need for intubation and invasive mechanical ventilation (MV) within 72 hours of surfactant administration in preterm neonates with RDS. The secondary objectives were the effects of these methods on the need for a second surfactant dose, mortality rate, and other preterm morbidities.
Methods
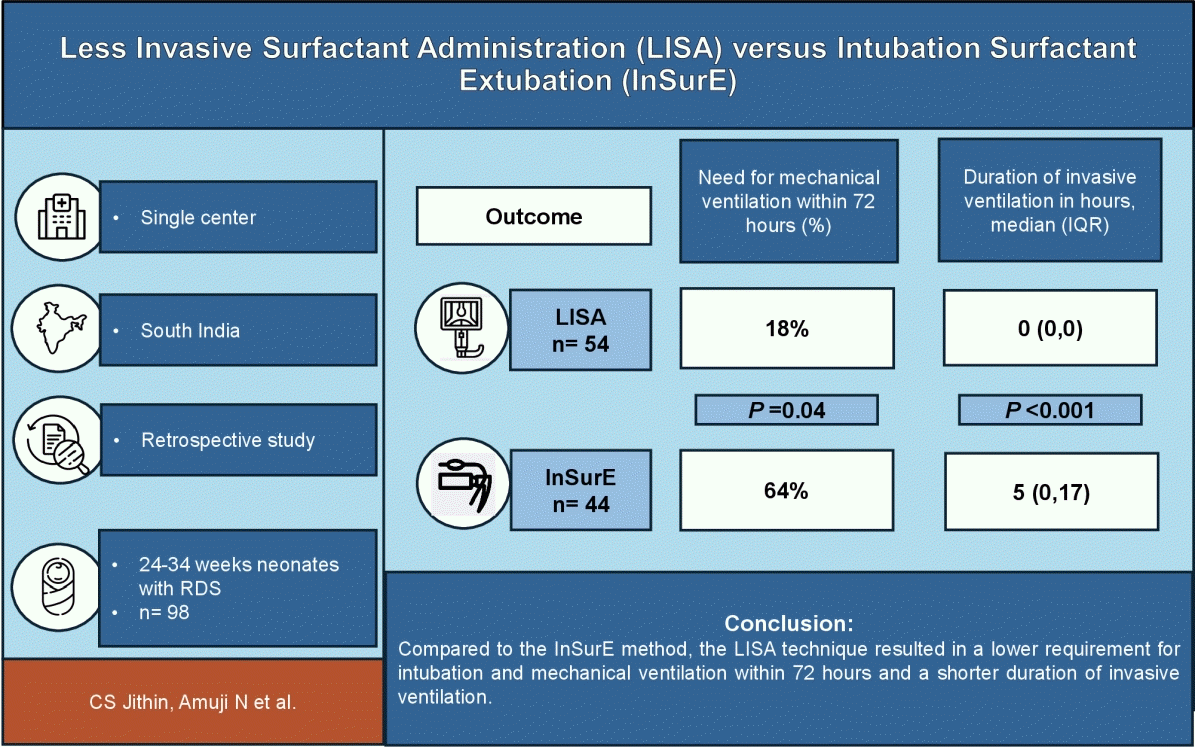
This retrospective observational study was conducted in Southern India over 5 years. Clinical outcomes were analyzed in neonates with RDS at 24–34 weeks' gestation who received surfactants via the LISA or InSurE method.
Results
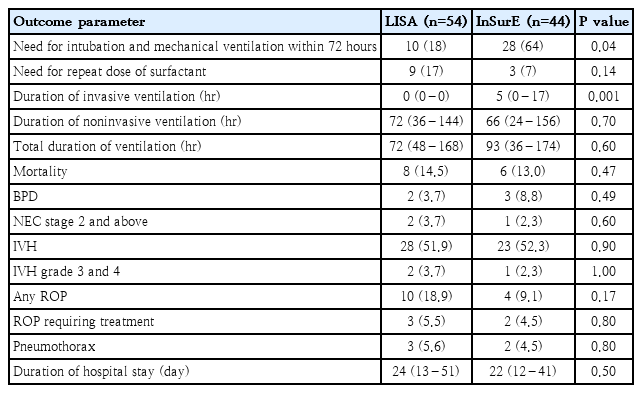
A total of 98 neonates were divided into the LISA group (n=54) and the InSurE group (n=44). The need for intubation and MV within 72 hours was significantly lower in the LISA versus InSurE group (18% vs. 64%, P=0.04; relative risk, 0.28; 95% confidence interval, 0.16–0.53). The duration of invasive ventilation was significantly shorter in the LISA group (P<0.001). We observed no significant intergroup differences in the need for a second surfactant dose (17% vs. 7%, P=0.14), bronchopulmonary dysplasia (3.7% vs. 8.8%, P=0.49), or mortality (14.5% vs. 13%, P=0.47).
Conclusion
LISA appears to be a less invasive and more effective alternative to InSurE, demonstrating the ability to reduce the need for intubation and invasive ventilation within the first 72 hours as well as the duration of invasive support in preterm infants with RDS.
Key message
Question: Does less invasive surfactant administration (LISA) (vs. intubation-surfactant-extubation) improve clinical outcomes in preterm infants with respiratory distress syndrome?
Finding: LISA significantly reduced intubation and invasive mechanical ventilation needs within the first 72 hours and shortened the overall invasive respiratory support duration without increasing other morbidities.
Meaning: LISA is a less invasive and safer surfactant delivery alternative. Larger multicenter trials are needed to confirm its long-term safety and efficacy, especially in low- and middle-income countries.
Graphical abstract. RDS, respiratory distress syndrome.
Introduction
Respiratory distress syndrome (RDS) due to surfactant deficiency remains a major cause of morbidity and mortality in preterm neonates. The main modality of treatment for RDS is early nasal continuous positive airway pressure (nCPAP) and selective use of surfactant which has been shown to reduce bronchopulmonary dysplasia (BPD) [1-3]. Mild cases of RDS are managed with CPAP alone, whereas moderate to severe RDS require exogenous surfactant therapy [1,4,5]. Over the years since the discovery of exogenous surfactants, various techniques have evolved for their administration. When surfactant therapy for RDS was first started, it involved intubation, surfactant administration, and subsequent continuation of mechanical ventilation (MV). To minimize ventilation-associated complications in preterm with RDS, the intubation-surfactant-extubation (InSurE) technique has been widely adopted. This method involves brief endotracheal intubation for surfactant administration, followed by prompt extubation to noninvasive respiratory support, thereby aiming to reduce the duration and risks associated with MV [6].
Consequently, the focus has been shifted to minimally invasive surfactant therapies. The less invasive surfactant administration (LISA) technique was one of them, which uses a thin catheter for surfactant instillation during spontaneous breathing, thus avoiding intubation [7]. Studies have demonstrated that LISA can effectively deliver surfactants with fewer side effects and improved outcomes compared to more invasive methods [3,8,9].
There are only a few studies from low-middle-income countries (LMICs) exploring the effectiveness of the LISA technique [10,11]. LISA method was introduced in our unit in 2018. Due to variable clinical responses observed, we conducted this study to determine the optimal surfactant administration strategy for preterm with RDS. The main aim of this study was to evaluate the effect of LISA versus InSurE on the need for intubation and MV within 72 hours of surfactant administration. We also compared the 2 methods in terms of the need for a second dose of surfactant, duration of ventilation, length of hospital stay, and the incidence of BPD, intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), pneumothorax, and mortality.
Methods
This retrospective observational study was conducted at a tertiary care hospital in Southern India over a 5-year period, from January 2018 to December 2022. All neonates between 24 and 34 weeks of gestation diagnosed with RDS and who received surfactant therapy via either LISA or InSurE technique were included. Neonates who were intubated in the delivery room, referred to other centers, or discharged against medical advice before the primary outcome could be assessed were excluded. Data was retrieved from the hospital Neonatal Perinatal Database and supplemented by individual case records obtained from the Medical Records Department. Approval and waiver of consent were granted by the Institutional Ethics Committee (IEC Reference Number: 133/2023).
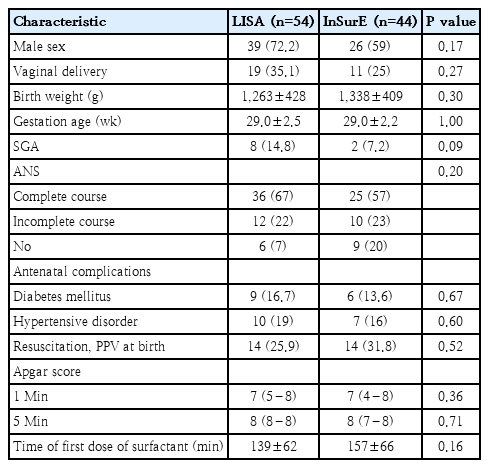
Demographic variables collected for both groups included gestational age, birth weight, sex, mode of delivery, Apgar scores at 1 and 5 minutes, small for gestational age (SGA) status, completion of antenatal corticosteroid therapy, and the interval between birth and surfactant administration. SGA was defined as birth weight below the 10th percentile for gestational age [12]. The primary outcome assessed was the need for intubation and MV within 72 hours of surfactant administration. Neonates requiring invasive ventilation for more than 4 hours were considered to have met the primary outcome [13,14]. The secondary outcomes measured were the need for a second dose of surfactant, the total duration of ventilation which was the sum of invasive and noninvasive ventilation periods, length of hospital stay, incidence of BPD [15], IVH [16], ROP [17], NEC [18], pneumothorax, and mortality. BPD was defined according to the 2018 National Institute of Child Health and Human Development criteria [15].
Surfactant administration followed the unit's standard protocol. The first dose was administered after obtaining parental consent when the infant's fraction of inspired oxygen (FiO2) requirement exceeded 0.3 with a positive end expiratory pressure (PEEP) of ≥6 cmH2O [1]. The choice between LISA and InSurE techniques was based on clinician preference and experience. Both procedures were performed by neonatology residents trained through simulation-based modules. Surfactants used included Survanta (25 mg/mL, Abbott, USA) and Neosurf (27 mg/mL, Cipla Pharmaceuticals, India). Doses were standardized according to phospholipid content: 100 mg/kg for survanta (beractant) and 135 mg/kg for neosurf (bovine lipid extract surfactant).
1. InSurE technique
In this study, the InSurE technique was administered to infants on noninvasive ventilation who met the criteria for surfactant therapy. These infants were intubated solely for surfactant administration and subsequently extubated to CPAP within a specified timeframe. As there was no universally accepted standard for the timing of extubation, we adopted a 2-hour threshold based on evidence from existing literature [13,14]. The primary outcome was met if the infant continued to require invasive MV beyond 2 hours after intubation. Surfactant was administered in 4 aliquots, with gentle bag ventilation provided between doses to facilitate distribution. Fentanyl was used for premedication only when clinically indicated, based on the attending physician’s judgment.
2. LISA technique
We followed a modified 'take care' method for LISA administration, wherein a 5Fr infant feeding tube was inserted orally into the trachea under direct laryngoscopy [19]. Details of the LISA technique is provided in Supplementary Material 1.
In both groups, a repeat dose of surfactant was administered 6 hours later if the FiO2 requirement was ≥0.4 [1]. Neonates were intubated if any of the following criteria were met: on CPAP of >7 cmH2O with FiO2>60% to maintain a target oxygen saturation (SpO2) between 91%–95%; partial pressure of carbon dioxide in arterial blood (PaCO2) >60 mmHg with pH <7.2; or if there were recurrent apneic episodes (>2 per hour), or apnea with bradycardia accompanied by hemodynamic instability [10,20]. Neonates were considered for extubation if they remained free from respiratory distress or apnea for at least 24 hours, while on the following ventilator settings: peak inspiratory pressure 12–13 cmH2O, PEEP 4–5 cmH2O, and FiO2 <40%. Eligible infants were then extubated to either noninvasive positive pressure ventilation (NIPPV) or nCPAP.
3. Sample size and statistical analysis
According to a prospective non-randomized pilot study by Ramos-Navarro et al. [21] the incidence of MV within 72 hours in the LISA group was 43.3%, and in the InSurE group was 73%. A sample size of at least 86 (43 in each group) was required for the study to have a power of 80% with a 95% confidence interval (CI). Qualitative data were presented as frequencies and percentages. Quantitative data were expressed as a mean with standard deviation (SD) or median with interquartile range (IQR). Pearson chi-square test (if samples >5) or Fisher exact test (if samples <5) was used to compare categorical variables like percentages across 2 groups. To compare numerical variables like birth weight (g) and gestational age (weeks) independent samples t tests were used. The Mann-Whitney test was used for nonnormal data. P value <0.05 was considered significant. Data analysis was performed using IBM SPSS Statistics ver. 19.0 (IBM Co., USA).
Results
Of the 729 neonates born between 24 and 34 weeks of gestation during the study period, 259 received surfactant therapy. However, the majority (n=158) were excluded as they were intubated in the labor room. A total of 98 neonates met the inclusion criteria and were enrolled in the study: 54 received surfactant via the LISA technique, while 44 received it via the InSurE technique (Fig. 1). The gestational age was 29.0±2.5 weeks (mean±SD) and 29.0±2.2 weeks in LISA and InSurE group respectively. The birth weight was 1,263±428 g (mean±SD) and 1,338±409 g in LISA and InSurE group respectively. The baseline characteristics were comparable between the 2 groups (Table 1).
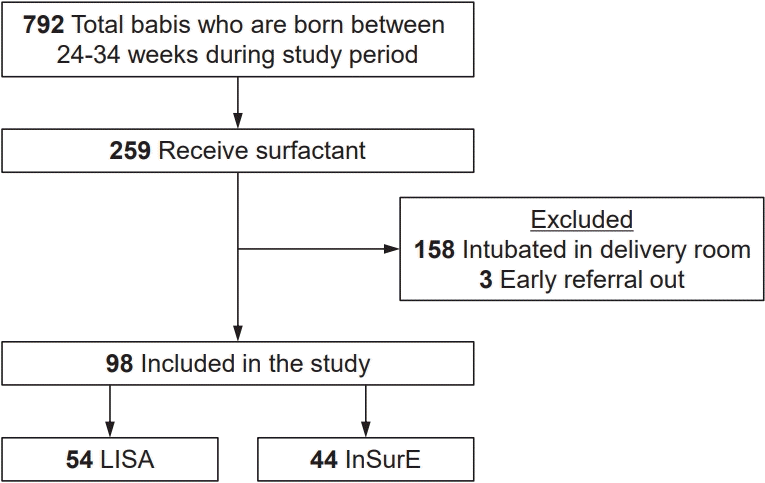
Study flow chart. LISA, less invasive surfactant administration; InSurE, intubation surfactant administration extubation.
The primary outcome: need for intubation and MV within the first 72 hours was significantly lower in the LISA group compared to the InSurE group (18% vs. 64%; P=0.04; relative risk, 0.28; 95% CI, 0.16–0.53). There were no statistically significant differences between the groups regarding secondary outcomes, including the need for repeat surfactant dosing, total ventilation days, duration of hospital stay, or incidence BPD, ROP, IVH, NEC, pneumothorax, or mortality. But overall requirement for invasive ventilation was also significantly reduced in the LISA group (P<0.001) (Table 2).
Discussion
In this retrospective study, we compared the LISA technique with the traditional InSurE method in preterm infants born between 24 and 34 weeks of gestation. The LISA group demonstrated a significantly lower need for intubation and MV within the first 72 hours of life (18% vs. 64%, P=0.04) along with a shorter duration of invasive ventilation compared to the InSurE group. No significant differences were observed between the 2 groups in terms of secondary outcomes, including the requirement for a second dose of surfactant, duration of ventilation, BPD, NEC, ROP, pneumothorax, duration of hospital stay, and mortality. These findings suggest that the LISA technique may offer a protective advantage by reducing the need for early invasive respiratory support, thereby potentially decreasing respiratory morbidity in this high-risk population.
Notably, most of the existing literature comparing LISA and InSurE originates from high-income countries which showed benefit in terms of reducing the need for MV. Kanmaz et al. [22] compared the 'take care' procedure using a 5Fr nasogastric catheter for tracheal catheterization with the InSurE method and reported that the need for MV within the first 72 hours was significantly lower with the LISA approach. (30% vs 45%, P=0.02). Dargaville et al. used a 16-gauge semirigid vascular catheter instead of feeding tube for intratracheal catheterization and reported a reduction in need of MV in first 72 hours in 25–28 weeks in infants (32% vs. 68%, P=0.00) but not in 29-34 weeks (22% vs. 45%, P=0.057) [23]. The results of German multicenter study revealed that in extremely premature (23–26 weeks gestation) infants in intervention group (LISA) had significantly less frequent intubation (74.8% vs. 99%, P=0.001) and fewer days on MV as compared to control group [24]. Our findings were consistent with these studies, and we also observed a 72% reduction in need of intubation and MV within 72 hours (18% vs. 64%, P=0.04; relative risk, 0.28; 95% CI, 0.16–0.53). These observations are further supported by multiple meta-analyses, which reported a consistent reduction in the need for invasive ventilation with the use of LISA [7,9,25,26].
In contrast, our results were not completely consistent with studies from LMICs. Studies from India by Jena et al. reported 51% (9% vs 40%, P<0.01) [27], and by Anand et al. [28] reported 50% reduction (9.5% vs. 25%, P=0.017) in the need of MV within 72 hours of birth. Similar results were seen in Pakistan by Halim et al. [29] (30% vs. 60%, P<0.05) and in Iran by Boskabadi et al. [30] (0% vs. 20%, P=0.027). However, studies from India by Gupta et al. [31], Mishra et al. [11], Pareek et al. [10], from Iran by Mohammadizadeh et al. [20] and Mosayebi et al. [32] and from China by Bao et al. [33] reported no significant reduction in the need for MV within 72 hours with the LISA method compared to the InSurE method. Key findings of all these studies are mentioned in Supplementary Table 1. Some of the speculated reasons for this discrepancy include differences in patient demographics and the use of NIPPV as the primary respiratory support modality. The proven benefits of NIPPV, such as better pressure delivery and a lower risk of intubation compared to nCPAP might have mitigated the added advantage of LISA in these studies [34]. In most studies showing a significant reduction in the need for MV, nCPAP was used as the primary mode of respiratory support following surfactant administration.
While our primary outcome demonstrated significant benefit with LISA, the secondary outcomes- such as duration of noninvasive ventilation, BPD, ROP, IVH, NEC, and pneumothorax did not differ significantly between the groups. In contrast, studies from high-income settings, including meta-analyses, have reported that LISA may reduce the incidence of BPD, severe IVH, and pneumothorax [25,35,36]. None of the studies from LMICs mentioned have demonstrated statistically significant differences in these preterm comorbidities [10,11,32,33]. This discrepancy may reflect the complex and multifactorial etiology of these conditions, influenced by a range of perinatal and postnatal factors beyond surfactant delivery techniques.
This study is one of the few conducted in LMICs to compare the LISA and InSurE techniques and demonstrate a significant difference in the primary outcome, thereby valuably contributing to the existing literature. A key strength of this study is the inclusion of infants with gestational ages as low as 24 weeks, allowing for a broader representation of the preterm population. Additionally, the extended study period of 5 years enhances the robustness and temporal relevance of our findings. However, several limitations must be acknowledged. The retrospective nature of the study inherently limits the ability to control for confounding variables. The sample size was relatively small, and the study was not adequately powered to detect differences in secondary outcomes. Immediate procedural complications could not be analyzed, as they were not consistently documented in the dataset. Additionally, the duration of surfactant administration for both LISA and InSurE techniques was not recorded, preventing comparison of procedural efficiency. Long-term outcomes, including neurodevelopmental follow-up, were also beyond the scope of this study due to its design.
In conclusion, our study suggests that the LISA method is a less invasive and effective alternative to the InSurE technique for surfactant administration in preterm infants under 34 weeks of gestation. Future research should focus on well-powered, prospective randomized controlled trials in LMIC settings to validate the short-term benefits and long-term outcomes, including neurodevelopmental impact. Studies comparing procedural efficiency, including duration and complications of surfactant administration, and evaluating the role of different noninvasive ventilation strategies postsurfactant, would further strengthen the evidence base and guide clinical practice in resource-limited environments.
Supplementary materials
Supplementary Material 1 and Supplementary Table are available at https://doi.org/10.3345/cep.2025.00332.
Details of the procedures
Summary of evidence from previous 10-year randomized controlled trials
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
We sincerely acknowledge all the parents, doctors, and nursing staff who contributed to the successful conduct of this study.
Author contribution
Conceptualization: AN, AS; Data curation: CSJ; Formal analysis: CSJ; Project administration: AN, AS; Writing - original draft: CSJ; Writing - review & editing: CSJ, AN, AS, PNSR