HLA‒B*58:01 and skin reactions in pediatric hematology and oncology patients treated with allopurinol
Article information
Abstract
Background
Allopurinol is widely used to prevent hyperuricemia in patients with tumor lysis syndrome. However, its use can trigger severe cutaneous adverse reactions (SCARs) with a mortality rate of approximately 11.39%. The human leukocyte antigen (HLA)–B*58:01 genotype is a major risk factor for SCARs. Although most studies to date have examined HLA–B*58:01 in Thai adults, data on pediatric patients are limited.
Purpose
Here we aimed to evaluate the association between HLA-B*58:01 and skin reactions in children with hematological or oncological diagnoses receiving allopurinol and determine its prevalence in this population.
Methods
Pediatric patients (age≤18 years) with hematological or oncological diseases who received allopurinol were enrolled in this cross-sectional study of previously exposed and newly prescribed cases. HLA-B*58:01 genotyping was performed to assess its association with skin reactions.
Results
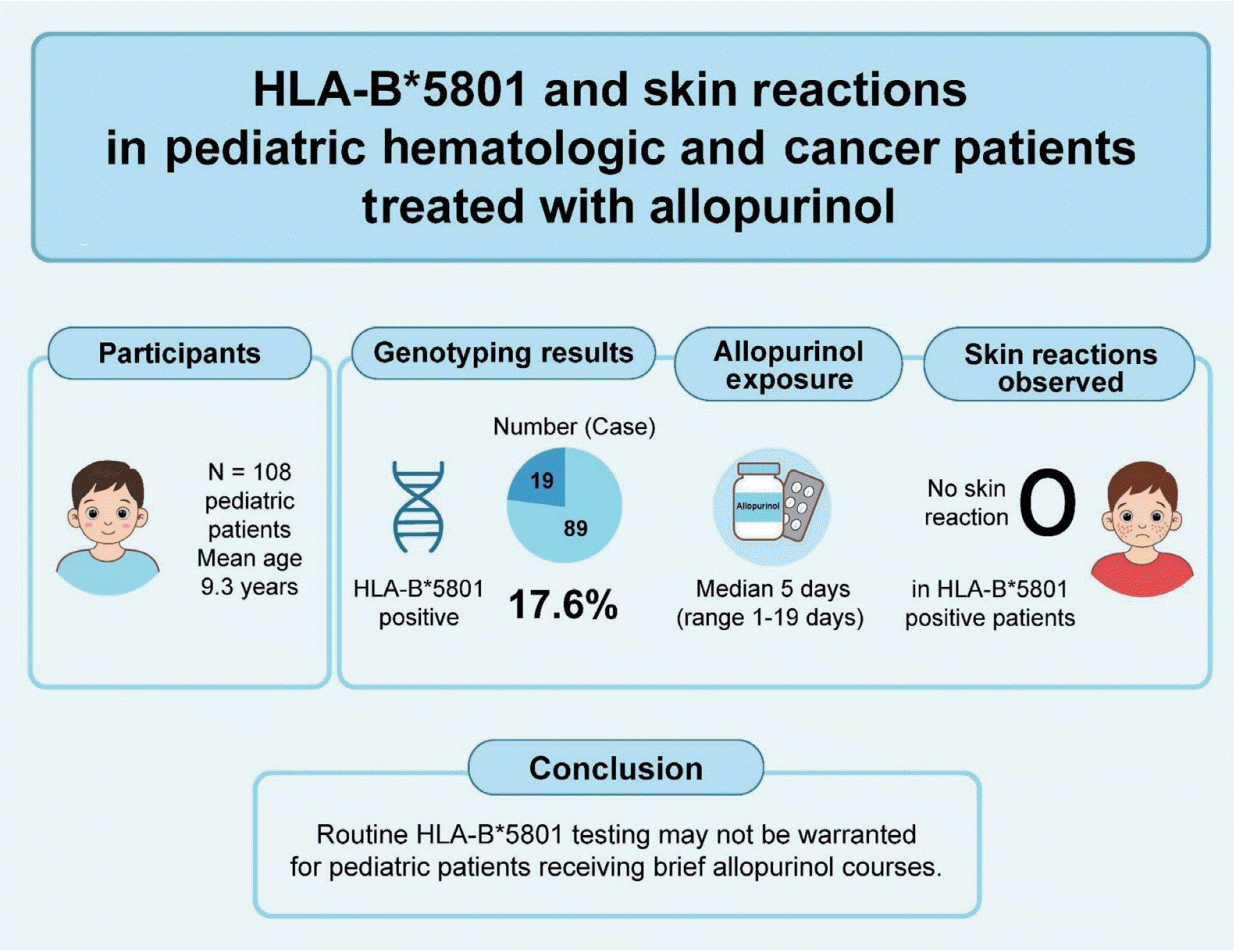
A total of 108 patients (mean age, 9.3 years) were included. Most patients (n=93, 86.1%) received allopurinol as prophylaxis for tumor lysis syndrome. Of them, 75 (69.4%) received allopurinol concomitantly with chemotherapy for malignancies, whereas the remaining patients received allopurinol during conditioning for hematopoietic stem cell transplantation. The prevalence of HLA–B*58:01 positivity was 17.6% (n=19 of 108 patients). The median exposure duration was 5 days (range, 1-19 days). No HLA–B*58:01–positive patients experienced a skin reaction. However, one patient who tested negative for HLA-B*58:01 developed a maculopapular rash on day 2 of the allopurinol therapy and required intravenous antihistamines.
Conclusion
Short-duration allopurinol exposure likely mitigates the risk of SCARs regardless of HLA–B*58:01 status. Routine HLA-B*58:01 testing may not be warranted in pediatric patients receiving brief allopurinol courses. However, larger studies are required to confirm these findings.
Key message
Question: Does human leukocyte antigen (HLA)–B*58:01 increase the risk of cutaneous reactions in pediatric patients with hematological and oncological diseases receiving allopurinol?
Finding: Of 108 patients, 17.6% carried HLA–B*58:01 but none developed skin reactions. The only rash occurred in an HLA-B*58:01–negative patient.
Meaning: Short-duration allopurinol may mitigate severe cutaneous adverse reaction risk regardless of genotype. Routine HLA-B*58:01 screening may be unnecessary in pediatric patients with hematological and oncological diseases briefly receiving allopurinol.
Graphical abstract
Introduction
Allopurinol is an essential medication included in the National List of Essential Medicines. It is highly effective for managing hyperuricemia and gout, and it prevents hyperuricemia related to tumor lysis syndrome. However, it can also induce cutaneous adverse drug reactions, ranging from mild maculopapular eruptions to life-threatening conditions. These include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis [1-3]. Severe cutaneous adverse reactions (SCARs) can cause significant morbidity and mortality [1-4]. Previous study investigating SCARs risk factors in the Thai population reported an 11.4% mortality rate among patients who developed severe hypersensitivity reactions [4]. Given these potentially fatal outcomes, preventing adverse reactions is paramount.
Multiple studies have identified various risk factors for allopurinol-induced SCARs, with genetic predisposition being the most significant risk factor [5]. Specifically, the human leukocyte antigen (HLA) allele HLA-B*58:01 is strongly implicated in SCARs development [3,6,7]. In the Thai population, this allele has a prevalence of about 15% [8,9], and carriers are at a markedly higher risk of severe hypersensitivity reactions than non-carriers [1-3]. Screening for HLA-B*58:01 allows clinicians to mitigate SCARs risk by identifying high-risk individuals prior to initiating allopurinol. This proactive strategy promotes patient safety and supports cost-effective public health measures [8,10,11].
According to clinical practice guidelines for pharmacogenetic testing of HLA-B*58:01 [12] and an announcement by the Thai National Health Security Board on May 7, 2021, HLA-B*58:01 screening is recommended for all new allopurinol users, as well as for patients who have taken allopurinol for fewer than 3 months without hypersensitivity reactions [13]. SCARs secondary to allopurinol can develop over variable exposure durations. In a case-control study across 4 European countries, 13 of 245 patients with SJS or TEN (5.3%) had received allopurinol, compared to 11 of 1147 control subjects who were hospitalized for other reasons (1%). The adjusted relative risk of allopurinol-associated SJS or TEN was 5.5 (95% confidence interval [CI], 2.0–15) [14]. While most HLA-B*58:01 prevalence studies focus on adults [8], pediatric data remain limited. Nevertheless, this information is crucial because allopurinol is first-line therapy for hyperuricemia in pediatric leukemia and lymphoma patients with tumor lysis syndrome. Additionally, in our practice, pediatric patients undergoing hematopoietic stem cell transplantation (HSCT) often receive allopurinol for 7-10 days during conditioning regimens to prevent hyperuricemia from cell lysis.
This study investigated the prevalence of HLA-B*58:01 and the incidence of allopurinol-induced cutaneous adverse reactions in pediatric patients with hematologic and oncologic diseases. By examining both previously treated and newly diagnosed patients, we aimed to evaluate the clinical utility of HLA-B*58:01 screening in this population.
Methods
1. Study design and ethical approval
This cross-sectional study included 108 pediatric patients (birth-18 years) diagnosed with hematologic or oncologic diseases who received allopurinol. Both previously treated patients and newly prescribed cases were enrolled after obtaining informed consent from the patients and their parents. The study aimed to investigate the association between HLA-B*58:01 genotype and cutaneous adverse reactions following allopurinol administration. Patients were recruited at the Faculty of Medicine Siriraj Hospital, Mahidol University. Ethical approval was obtained from the Ethics Committee of the Faculty of Medicine Siriraj Hospital (reference: Si-083/2024).
2. Participants and setting
Eligible patients were aged 0-18 years, of Thai nationality, and had hematologic or oncologic diagnoses requiring allopurinol therapy. Patients who were not Thai nationals were excluded. The study population included those receiving allopurinol for any duration, whether newly prescribed or previously exposed.
3. HLA‒B*58:01 genotyping
Blood samples (1-3 mL) were collected during routine laboratory procedures. HLA-B*58:01 genetic testing was performed at the Molecular Genetics Laboratory, Siriraj Center of Genomics, Faculty of Medicine Siriraj Hospital. Detection of HLA-B*58:01 associated allopurinol hypersensitivity was performed using melting-temperature analysis by real-time polymerase chain reaction (PCR). An in-house PCR-SSP (sequence-specific primer) method and a modified reverse primer with an introduced mismatched base near the 3′ end was applied to specifically detect the HLA-B*58:01 allele and exclude HLA-B*57:01 in genomic DNA. Real-time PCR was performed using a PCRmax Eco48 system (Coleparmer, USA), with EvaGreen dye (Biotium, USA) as the fluorescent reagent.
4. Data collection
Demographic data, HLA-B*58:01 genotyping results, and details of allopurinol use (dosage and duration) were recorded. Hyperuricemia defined as uric acid ≥ 8 mg/dL. Skin reactions were monitored and classified as maculopapular rash, SJS, TEN, DRESS, or acute generalized exanthematous pustulosis. Onset, severity, and treatment of any adverse reactions were documented. Concomitant medications were also recorded to assess potential drug interactions.
5. Statistical analysis
Descriptive statistics were used to summarize the data. Continuous variables were reported as means with standard deviations or medians with ranges (interquartile range, IQR), depending on the distribution. Categorical variables were presented as frequencies and percentages. Prevalence was expressed as a percentage with a 95% confidence interval. Associations between patient characteristics and HLA-B*58:01 genotype were examined using the chi-square test, 2-sample t test, or Mann-Whitney U test, as appropriate. All statistical analyses were conducted using IBM SPSS Statistics ver. 22.0 (IBM Co., USA). A P value <0.05 was considered statistically significant.
Results
1. Baseline characteristics
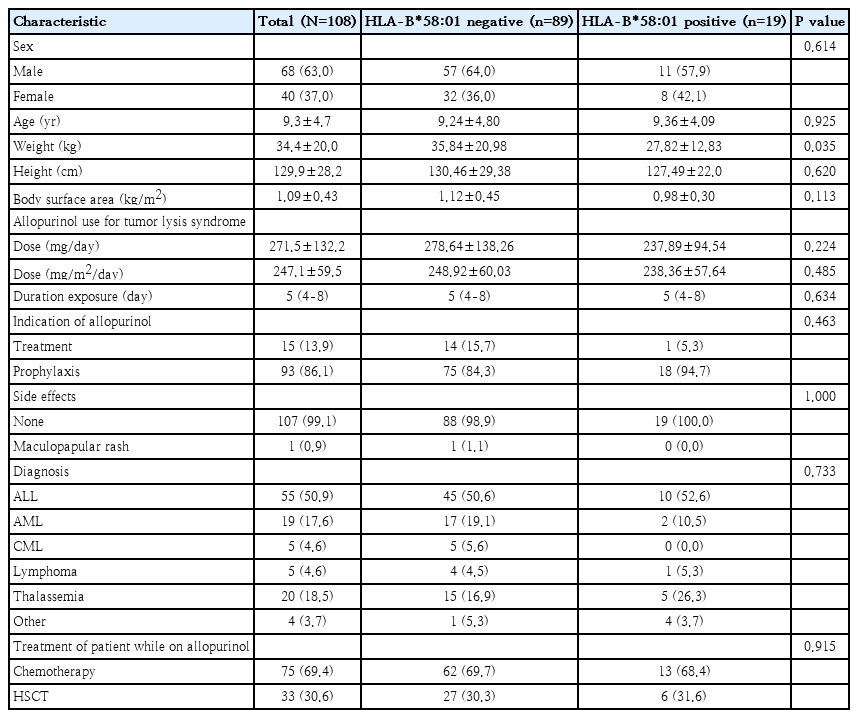
A total of 108 pediatric patients diagnosed with hematologic and oncologic diseases were enrolled. The mean age was 9.3 years, and 63.0% were male. Among the participants, 19 (17.6%) tested positive for HLA-B*58:01. There were no significant differences in age or sex distribution between HLA-B*58:01-positive and -negative groups. However, those carrying HLA-B*58:01 had a significantly lower mean weight than non-carriers (P=0.035), while height and body surface area did not differ significantly. The most common diagnosis was acute lymphoblastic leukemia (ALL; 50.9%), followed by transfusion-dependent thalassemia (18.5%) and acute myeloid leukemia (17.6%). Disease distribution was not different between both groups (P=0.733).
Most patients (69.4%) received allopurinol concomitant with chemotherapy for treatment of malignancies, while 30.6% received allopurinol during the conditioning regimen for HSCT. The proportion of patients in these 2 treatment settings did not significantly differ between HLA-B* 58:01-positive and -negative groups (P=0.915). Detailed baseline characteristics and genotypic distribution are shown in Table 1.
2. Indications for allopurinol
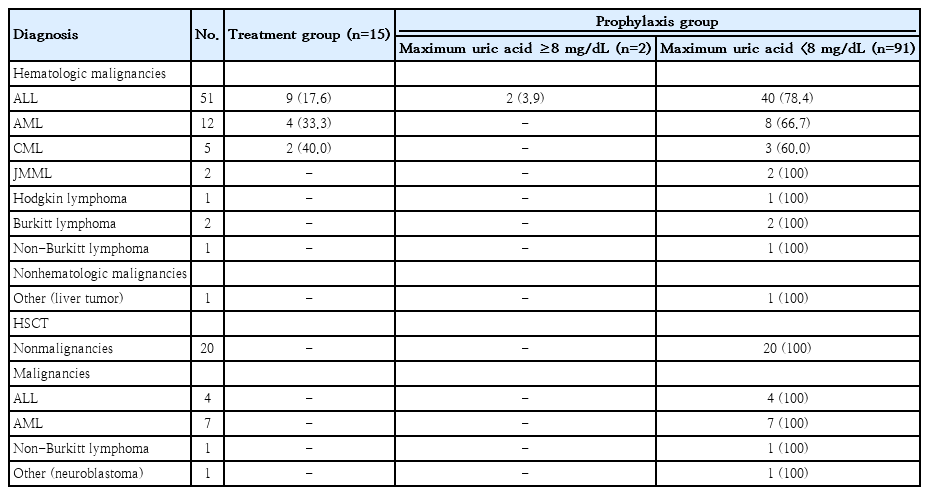
To evaluate the necessity of initiating allopurinol, patients were categorized according to their baseline uric acid levels to assess the appropriateness of prophylactic versus therapeutic use. As summarized in Table 2, 15 patients (13.9%) had uric acid levels ≥8 mg/dL and received allopurinol for the treatment of hyperuricemia, while the remaining 93 patients (86.1%) were prescribed allopurinol primarily for tumor lysis syndrome prophylaxis. Of these 93 patients, 2 (2.2%) developed hyperuricemia while on allopurinol prophylaxis. Patients in the treatment group received intravenous hydration concomitantly with allopurinol (median duration, 8 days; IQR, 5-8 days; overall range, 2–12 days). Whereas, patients in the prophylaxis group received allopurinol with median duration 5 days (IQR, 4-8 days; overall range, 1–19 days). Among HLA-B* 58:01-positive patients, 94.7% received allopurinol for prophylaxis, compared with 84.3% among those who were HLA-B*58:01-negative (P=0.463). The median duration of allopurinol exposure was 5 days (IQR, 4-8 days; overall range, 1-19 days) in both groups. The mean allopurinol dose was 247.1±59.5 mg/m2/day, with no significant difference between genotypes.
In patients with hematologic malignancies (ALL, acute myeloid leukemia, and chronic myeloid leukemia), prophylactic use was predominant, especially in ALL (82.3%). Only 2 patients with ALL (2.2% of the 93 prophylaxis recipients) had elevated uric acid levels (≥8 mg/dL) after starting allopurinol. All HSCT patients received allopurinol for prophylaxis, and none developed hyperuricemia. The indications for allopurinol therapy by diagnosis and uric acid levels were summarized in Table 2.
3. Cutaneous adverse reactions
In our study, only 1 patient (0.9%) in HLA-B*58:01-negative group developed a maculopapular rash on the face and forearms on day 2 of allopurinol exposure, necessitating intravenous antihistamines. This patient was also receiving gentamicin, phenytoin, and busulfan during allopurinol use. None of HLA-B*58:01-positive patients experienced cutaneous adverse reactions. However, one HLA-B*58:01-positive patient with Hodgkin lymphoma with bulk disease initially received allopurinol for 2 days during the first chemotherapy course for prophylaxis hyperuricemia. Upon recurrence of hyperuricemia in the second course, allopurinol was replaced with febuxostat to mitigate the risk of SCARs.
4. Uric acid levels and treatment outcomes
In the treatment group (N=15), the mean baseline uric acid level was 9.7±1.8 mg/dL, which rose slightly to a peak of 9.8±1.8 mg/dL within 1 day. In the prophylaxis group (N=93), the mean baseline uric acid level was lower (5.0±1.6 mg/dL) and increased mildly to 5.2±1.6 mg/dL, with a peak at 1.3±0.8 days after allopurinol initiation. These findings underscore the predominant use of allopurinol for tumor lysis syndrome prevention and suggest that brief therapy may effectively control uric acid levels without a notable risk of SCARs.
Discussion
This study investigated the association between HLA-B* 58:01 genotype and cutaneous adverse reactions in pediatric patients with hematologic and oncologic diseases receiving allopurinol. The prevalence of HLA-B*58:01 in this cohort was 17.6%, consistent with earlier studies reporting approximately 15% in the Thai population [8,9]. However, despite the established link between HLA-B*58:01 and allopurinol-induced SCARs [3], none of the HLA-B*58:01-positive patients in this study developed hypersensitivity cutaneous reactions. This finding diverges from prior adult studies, which showed significantly increased risks of SCARs, including SJS, TEN, and DRESS, among HLA-B*58:01 carriers [1-3]. The absence of SCARs in our studies might explains by the fact that short exposure time (median duration of allopurinol exposure 5 days; IQR, 4-8 days) was least likely to cause hypersensitivity cutaneous reactions. This observation suggests that brief exposure to allopurinol may not pose a substantial SCARs risk, even in genetically susceptible individuals. Moreover, the turnaround time for HLA–B*58:01 testing at our institution is approximately 7 days, which may exceed the timeframe in which allopurinol is typically initiated. As a result, routine preemptive screening may be impractical in urgent clinical settings where allopurinol must be started immediately. Given the comparable allele prevalence and clinical context, our findings may be generalizable to pediatric hematology-oncology practices throughout Thailand, especially regarding the selective use of HLA–B*58:01 screening in patients receiving short-term allopurinol.
The association between HLA-B*58:01 and allopurinol-induced SCARs is extensively documented in adult populations. Some studies have reported odds ratios as high as 579 for SJS/TEN and 430 for DRESS in HLA-B*58:01 carriers [1-3]. The mean interval from allopurinol initiation to onset of SCARs in Thai individuals was reported 18–29 (range, 2–60) days [1,3,4]. Furthermore, adverse reactions to allopurinol have exhibited a dose-dependent relationship, with higher doses linked to an increased risk of SJS and TEN [15,16]. In this study, the pediatric cohort had a shorter exposure duration (median, 5 days) than the mean interval time to onset of SCARs reported after allopurinol initiation in other studies [1,3,4]. Most participants (86.7%) in our study received allopurinol for tumor lysis syndrome prophylaxis. In contrast, conditions like gout, a common cause of hyperuricemia in adults, often require prolonged allopurinol therapy [17]. This limited exposure window may explain the lack of observed SCARs, as previous research suggested that prolonged allopurinol use is a key risk factor for hypersensitivity reactions [5]. These findings suggest that SCARs risk may be exposure-dependent. They reinforce the hypothesis that HLA-B*58:01-mediated hypersensitivity requires a prolonged exposure period to manifest clinically. HLA–B*5801 testing may be selectively considered in patients expected to require prolonged or repeated allopurinol therapy, such as those with newly diagnosed acute leukemia with hyperleukocytosis, relapsed leukemia or lymphoma with hyperuricemia, or those with a high tumor burden at risk for recurrent hyperuricemia during subsequent courses of chemotherapy.
Routine HLA-B*58:01 screening is recommended in adult populations because of the high risk of SCARs from prolonged allopurinol therapy in majority of patients. However, the findings from this study suggest that preemptive genetic testing may not be essential before starting allopurinol in pediatric patients with short-term exposure. Nevertheless, HLA-B*58:01 screening remains clinically relevant in selected pediatric cases prone to long-term allopurinol therapy or recurrent hyperuricemia. This approach may be particularly important in patients with bulky lymphoma who could require re-exposure to allopurinol as observed in this study.
Despite the low incidence of hypersensitivity reactions in this study, the results should be interpreted cautiously due to the limited sample size. Larger, multicenter cohort studies are needed to clarify the safety of allopurinol use in pediatric setting, especially in those requiring recurrent or prolonged therapy. Future research should also explore other genetic and clinical risk factors for allopurinol-induced SCARs beyond HLA-B*58:01. Additional immune-mediated pathways may contribute to hypersensitivity in susceptible populations.
In conclusion, this study found no association between HLA-B*58:01 and allopurinol-induced cutaneous adverse reactions in pediatric patients receiving short-term therapy, most likely because of the brief duration of drug exposure. Consequently, routine HLA-B*58:01 screening may not be necessary before initiating allopurinol in pediatric patients who require only short-term treatment courses. However, genetic testing may be beneficial for patients at risk of recurrent hyperuricemia or those requiring repeated or prolonged allopurinol exposure, given that prolonged treatment duration increases the risk of SCARs. Larger, multicenter studies are needed to further clarify the role and cost utility of HLA‒B*58:01 testing in pediatric hematology and oncology, thereby guiding safe allopurinol use in this population.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research project was supported by a research grant from the Siriraj Research Development Fund, Grant number (IO) R016732020, Faculty of Medicine Siriraj Hospital, Mahidol University.
Acknowledgments
The authors gratefully acknowledge Assistant Professor Chulaluk Komoltri of the Research Department, Faculty of Medicine Siriraj Hospital, for her advice on statistical analysis, Ms. Nattha Yongwattana of the Molecular Genetics Laboratory, Siriraj Center of Genomics, Faculty of Medicine Siriraj Hospital, for her valuable contribution to the HLA–B*58:01 genetic testing, and Ms Onuma Sopuht of the Department of Pediatrics, Faculty of Medicine Siriraj Hospital, for her assistance with patient listing.
Author contribution
Conceptualization: JB, KS; Data curation: PM, CR; Formal analysis: PM, JB, KS; Funding acquisition: KS; Methodology: PM, JB, KS; Writing - original draft: PM, JB; Writing - review & editing: CR, JB, KS