Effects of phytoestrogen on sexual development
Article information
Abstract
Phytoestrogen is an estrogenic compound that occurs naturally in plants. The most common sources of phytoestrogen are soybean products, which contain high levels of isoflavones. This compound, which has structural similarity with estrogen, can act as an estrogen receptor agonist or antagonist. Animal studies provide evidence of the significant effects of phytoestrogen on sexual development, including altered pubertal timing, impaired estrous cycling and ovarian function, and altered hypothalamus and pituitary functions. Although human studies examining the effects of phytoestrogen on sexual development are extremely limited, the results of some studies agree with those of the animal studies. In this paper, we review the possible mechanism of phytoestrogen action and the evidence showing the effects of phytoestrogen on sexual development in animal and human studies.
Introduction
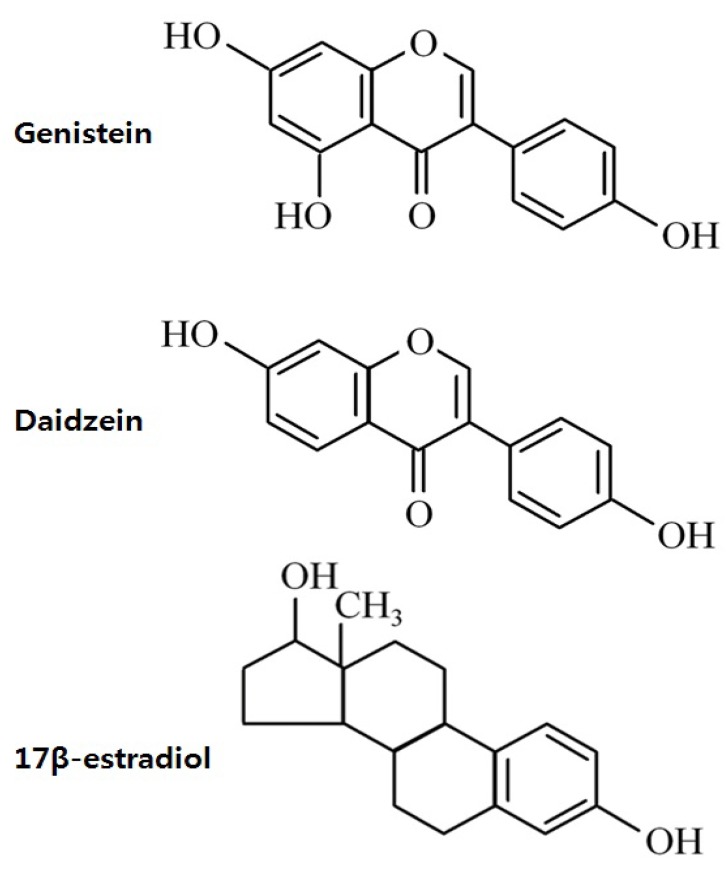
Phytoestrogen is a plant-derived compound that mimics estrogen1). Phytoestrogens can be classified as isoflavones, coumestans, and lignans (Fig. 1). Isoflavones, which are the most extensively studied phytoestrogens, are found predominately in soybeans and soy product-containing foods2). The major isoflavones are genistein and daidzein, and their conjugates account for greater than 65% of the total isoflavones3).
Phytoestrogen has significant binding affinity to estrogen receptor (ER), although much weaker than that of estradiol. Experimental animal studies have demonstrated several adverse effect of isoflavones on the reproductive system, such as premature pubertal onset4,5), reduced fertility6,7), altered estrous cyclicity5,8), and disrupted pituitary responsiveness to gonadotropin releasing hormone (GnRH)9). Therefore, concerns over the possible adverse effects of phytoestrogens on reproductive health have been raised in humans as well.
Studies on the hormonal effects of isoflavones are especially important in Koreans because soy products are a major component of the Korean diet; in fact, the consumption of an isoflavone-containing diet such as soy-based infant formula and soy-fortified foods and beverages has recently increased. In this paper, we review the possible mode of actions of phytoestrogen and summarize the results of animal and human studies showing the effects of phytoestrogens on sexual development, especially in childhood.
Mode of phytoestrogen action and target tissues
Estrogens act through 2 subtypes of their receptors found in target tissues: ERα and ERβ. Both ERs are widely expressed in various mammalian tissues; however, there are some differences in their distribution. ERα is most abundant in the uterus, and smaller quantities are detected in the ovaries, Leydig cells of the testes, female mammary glands, bones, and hypothalamus10,11). ERβ expression is highest in ovarian granulose cells and the gastrointestinal tract, whereas it is moderate to low in spermatids, the prostate, epididymis, female mammary glands, hypothalamus, and pituitary gland11,12).
The structure of isoflavones is closely similar to that of estrogens13). The presence of the 2 phenolic rings in isoflavones enables these compounds to bind to ERs (Fig. 2). Isoflavones exert estrogenic effects on a number of target organs that possess ERα and/or ERβ. Although the binding affinities to ERα and ERβ of isoflavones is much lower than those of 17β-estradiol, they can compete with 17β-estradiol for binding to the ERα and ERβ1). The binding affinity of genistein for ERβ is 87%, while that for ERα is 4%, while that of daidzein for ERβ and ERα is 0.5% and 0.1%, respectively14). The effect of phytoestrogen has been demonstrated to be either agonistic or antagonistic depending on its own concentrations15) or the estrogen concentrations of the environment16). At low concentrations, genistein appears to act as an agonist, stimulating the proliferation of estrogen-dependent breast cancer cells, whereas high concentrations inhibit estradiol-stimulated cell growth17). Hwang et al.16) showed that isoflavone metabolites act as ER agonists in a low-estrogen environment (postmenopausal level of estradiol) but act as ER antagonists in a high-estrogen environment (premenopausal level of estradiol).
Phytoestrogen exposure and sexual development in animals
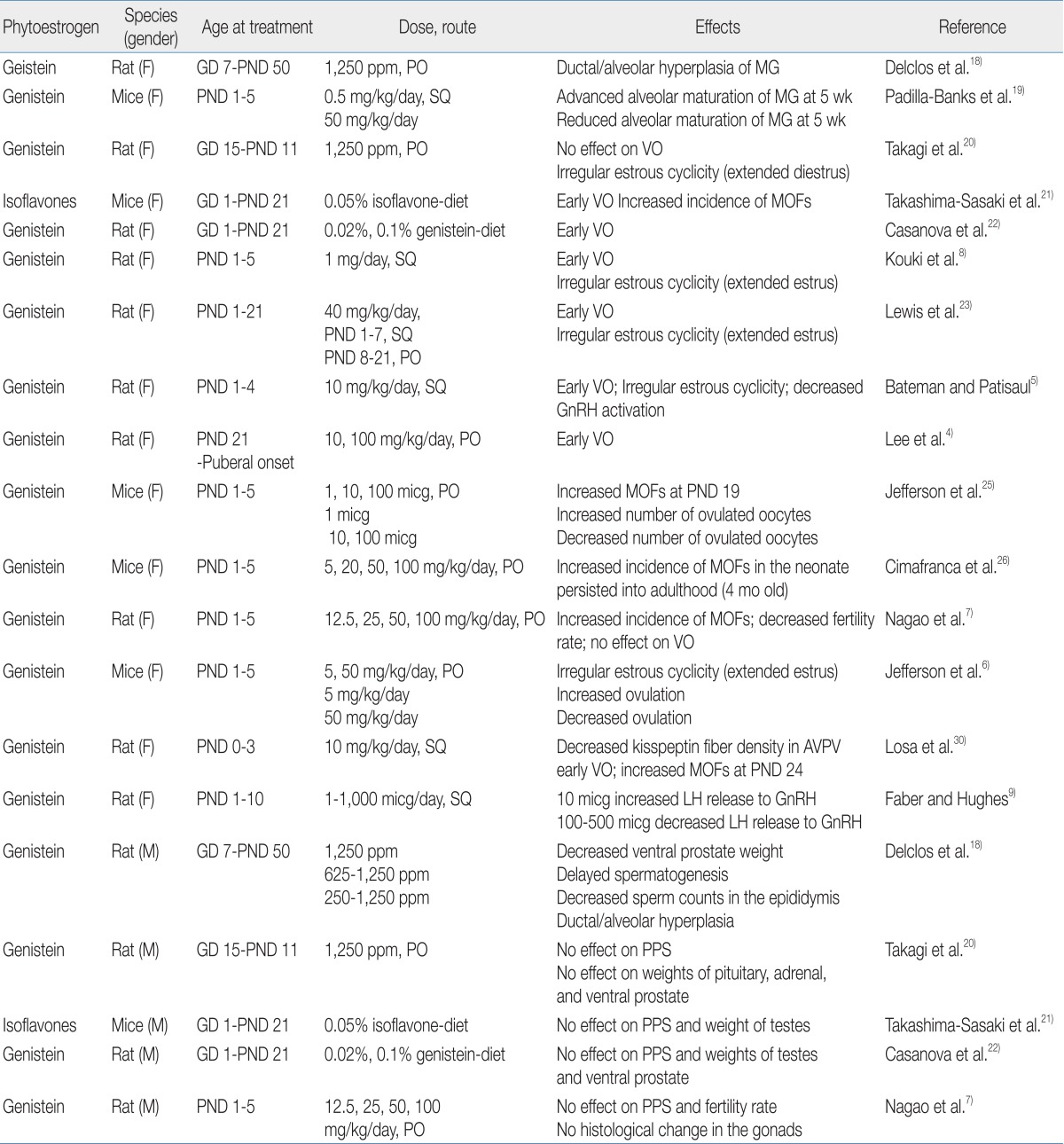
A number of animal studies have shown the adverse effects of phytoestrogens on reproductive organs (Table 1). Exposure to genistein affects mammary gland development and differentiation in rodents. Chronic genistein exposure from gestation day (GD) 7 to postnatal day (PND) 50 has been shown to result in ductal/alveolar hyperplasia of the mammary gland in pups18). Neonatal female mice treated with genistein showed a differential effect on mammary gland growth according to genistein dosage: low dose-treated mice exhibited advanced maturation with increased ductal elongation, whereas high dose-treated mice showed reduced lobular alveolar development19).
In the aspect of pubertal onset, the effects of phytoestrogens are quite different according to exposure time, dosage, and route. Signs of pubertal onset in rats/mice are vaginal opening in females and preputial separation in males. One study demonstrated that perinatal exposure to genistein did not affect the age of pubertal onset in male and female rats20). However, other studies found that dietary exposure to high doses of isoflavone from GD 1 to PND 21 induced the acceleration of vaginal opening in female offspring but did not affect preputial separation in male offspring21,22). Short-term exposure to high doses of isoflavone in the neonatal period has also been shown to expedite vaginal opening in female rats5,8,23). In fact, we recently found that exposure to high doses of genistein in the prepubertal period (from PND 21 until puberty) induces early vaginal opening in female rats4).
The adverse effects of genistein exposure on the developing ovaries have been shown in rodent studies. At birth, mice have large oocyte nests, and during the first week of life, these oocyte nests dissociate into individual oocytes surrounded by granulose cells24). This ovarian differentiation process requires a postpartum decrease in estrogen and progesterone24). Neonatal treatment with estrogen compounds such as 17β-estradiol and genistein disrupts this process, leaving the oocytes together in nests and leading to multi-oocyte follicles (MOFs)7,25,26). Since MOF-derived oocytes have been observed to have reduced fertility rates during in vitro fertilization27), an increased number of MOFs is one possible explanation for infertility in genistein-treated rodents28).
Numerous studies have shown that exposure of rodents to genistein in the neonatal period caused abnormal estrous cyclicity5,7,8) and altered ovulation6,25). These phenomena are mainly explained by the effect of genistein on the hypothalamic-pituitary-gonadal (HPG) axis, upon which normal estrous cyclicity is dependent6) and from which a mid-cycle surge of luteinizing hormone (LH) for ovulation is derived29). The response of the HPG axis in the neonatal period to genistein exposure seems to be dose-dependent. One study demonstrated that exposure to low doses of genistein in the neonatal period increased GnRH-induced LH release, whereas a high dose of genistein decreased GnRH-induced LH release9). Similarly, mice treated neonatally with low doses of genistein showed increased LH production and enhanced ovulation6,25), whereas rats exposed to high doses of genistein in the neonatal period demonstrated decreased GnRH activation and kisspeptin fiber density in the hypothalamus5,30). These data suggest that exposure to genistein in the neonatal period disrupts HPG function in a dose-dependent manner.
Relatively few studies have demonstrated the effects of phytoestrogen on male sexual development in rodents. Delclos et al.18) showed decreased ventral prostate weight and delayed spermatogenesis in male rats after genistein exposure from conception to puberty. However, exposure for short duration (GD 1 to the prepubertal period) did not affect pubertal timing, gonadal weight/histology, or fertility rate in male rodents7,20-22).
Phytoestrogen exposure and sexual development in children
Human phytoestrogen exposure is highly dependent on the consumption of soy-based foods. The traditional Asian diet includes greater amounts of soy products such as tofu, soy sauce, and tempeh than the typical Western diet; therefore, the Asian population has higher concentrations of isoflavone in the urine and plasma than the Western population31-33). Because phytoestrogens cross the placenta, fetuses are exposed to them through maternal dietary consumption34). After birth, infants are exposed to phytoestrogens though breastfeeding; however, the most extensive exposure to phytoestrogens occurs via soy-based formula feeding35).
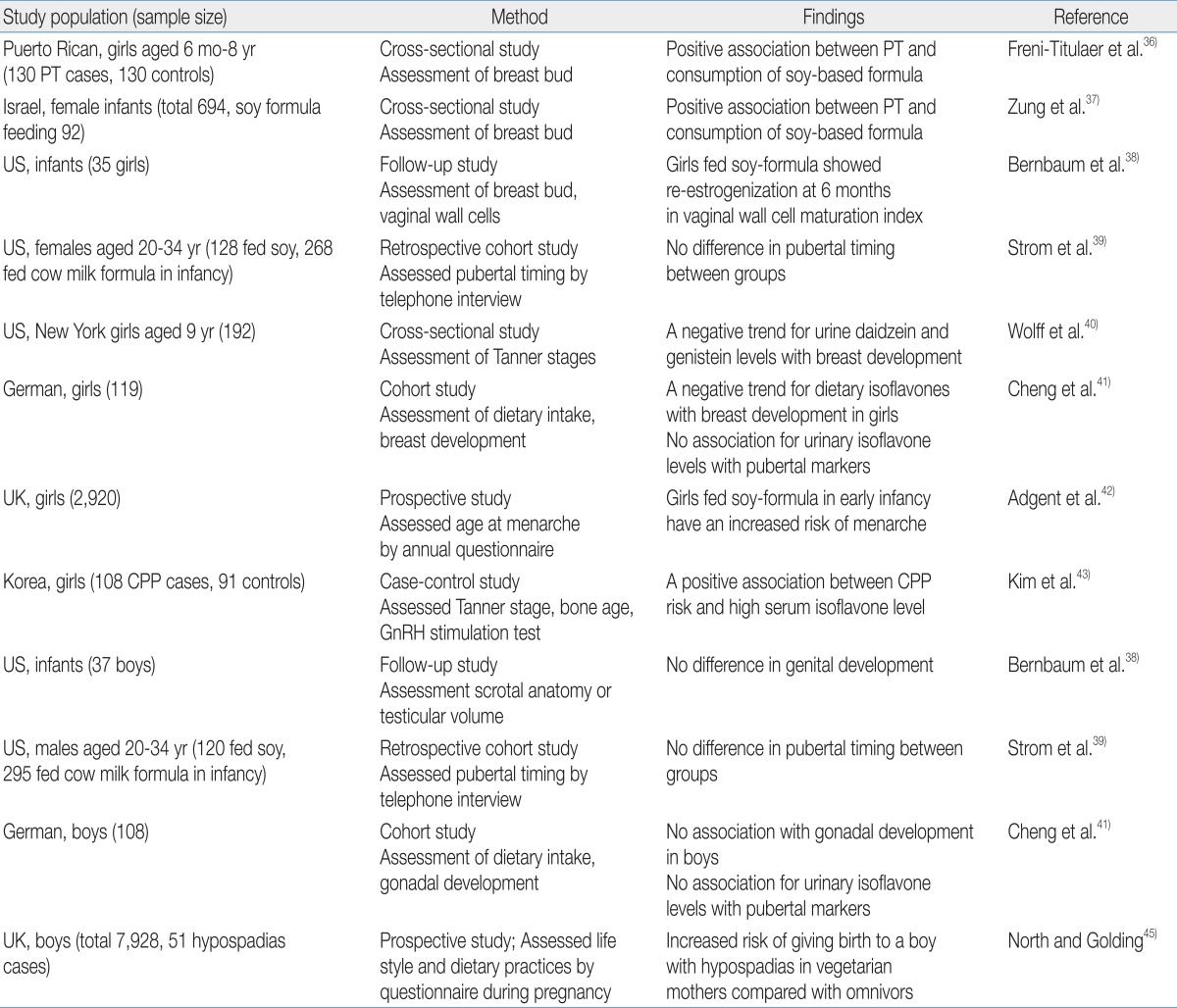
A few studies have investigated the relationship between phytoestrogen exposure and sexual development in children, and an association was mainly reported in girls (Table 2). Neonatal consumption of soy-based formula was independently associated with premature thelarche in Puerto Rican girls36). Zung et al.37) also reported that premature thelarche was more prevalent among soy formula-fed infants. In addition, the estrogenic activity of soy-based formula in infants involves vaginal cell maturation38). Vaginal wall cells show maximal maturation index at birth due to maternal estrogen and then rapidly lose the estrogen effect by the age of 1 month. This low maturation index is maintained in breast milk- or cow-based formula-fed infants; in contrast, the vaginal maturation index rises again in infants fed soy-based formula by the age of 6 months, indicating the estrogen activity of soy-based formula.
There are conflicting results in regard with pubertal timing. A telephone survey of young adults who had been fed soy formula during the infancy reported no significant changes in pubertal maturation39). Two studies reported that phytoestrogen exposure was associated with delayed puberty in girls. A cross-sectional study of 192 healthy 9-year-old girls found that delayed breast development was observed in girls with high concentrations of urinary daidzein and genistein40). A prospective cohort study reported that high isoflavone intake was associated with delayed breast development in German girls41). On the other hand, recent studies support that phytoestrogen exposure may induce earlier pubertal maturation. A prospective longitudinal study that enrolled pregnant women in the United Kingdom reported that early soy-fed infants had earlier menarche compared with girls fed breast milk or other types of formula42). We recently demonstrated that high serum isoflavone levels are associated with central precocious puberty in Korean girls43).
These conflicting results reflect the complex influence of phytoestrogens on the HPG axis. The effects of isoflavones on puberty seems be dependent on several factors, such as the amount of isoflavones consumed, timing of isoflavone exposure, and endogenous estrogen status of individuals. As discussed earlier, genistein exposure alters GnRH activation and pituitary responsiveness; specifically, a low dose of genistein increased GnRH-induced LH release, whereas a high dose of genistein decreased GnRH-induced LH release9). In the peripubertal period when endogenous estradiol levels are low, phytoestrogen exposure could induce increased sensitivity of the pituitary gland to GnRH challenge9), whereas exposure after pubertal onset could interfere with gonadotropin release as demonstrated in premenopausal women44).
For boys, one epidemiologic study found that maternal vegetarian diets containing large amounts of isoflavones are associated with hypospadias in male offspring45). However, most studies failed to show a significant association between phytoestrogen exposure and pubertal onset39,41).
Summary
Several animal studies have demonstrated significant effects of phytoestrogen on sexual development, including altered pubertal timing, impaired estrous cyclicity and ovarian function, and altered function of the HPG axis. Human studies are also examining the possible effects of isoflavone exposure on sexual development in childhood. Especially during infancy, which is a critical period for growth and development, soy formula feeding may exert estrogenic effects on multiple organs including the mammary glands, gonads, and brain. In human studies, it is difficult to absolutely control other factors influencing clinical outcomes, such as diet, genetic factors, or other environmental disruptors. Further prospective human studies are needed that control these factors as much as possible to reveal the potential health effects of soy-based formulas or other sources of phytoestrogens on sexual development in children.
Acknowledgment
This work was supported by a grant 09182KFDA600 from the Korea Food & Drug Administration in 2010.