Neuro-Behçet disease presented diplopia with hemiparesis following minor head trauma
Article information
Abstract
Behçet disease (BD) is rare in childhood. We report a 9-year-old boy with neuro-Behçet disease who presented diplopia and weakness on the left side after a cerebral concussion. Brain magnetic resonance imaging (MRI) revealed hyperintensity of the right mesodiencephalic junction on T2-weighted and fluid attenuated inversion recovery images. Prednisolone administration resulted in complete remission and normalization of abnormal MRI finding. Brain MRI is a useful diagnostic tool when the neurological sign is the first symptom of subclinical BD.
Introduction
Behçet disease (BD) is characterized by recurrent aphthous oral and genital ulcerations, uveitis, and systemic inflammation. This disease may include skin, joints, gastrointestinal tract, central nervous system (CNS), and other organs1). The nervous system involvement is one of the most serious manifestations of BD, leading to headache, confusion, paresis, cranial nerve palsy, cerebellar ataxia, or meningeal irritation signs. Neurological complications occur in about 5 to 25% of all patients with BD2). Neuro-Behçet disease (NBD) may be difficult to be diagnosed in patients without full-blown feature of BD. We present a case of child with NBD, in whom signs and symptoms suggest CNS involvement after brain concussion. Here we discuss the triggering factors that develop the typical symptoms of NBD in patients who do not fulfill criteria for BD (International Study Group for Behçet disease, 1990) (Table 1)3). Furthermore, we emphasize the value of magnetic resonance imaging (MRI) findings in the diagnosis of NBD.
Case report
A 9-year-old boy was transferred to our department in March 2007 with diplopia and left sided weakness after minor head injury. He was previously healthy and had no history of neurological disease. While playing, he was pushed by a boy and fell down on the ground by the back side of his head. He was not unconscious or disorientated but complaining of headache for some minutes and fell asleep. Next morning, sixteen hours later, he was found to have diplopia and left sided weakness. He had recurrent oral aphthous ulcer and intermittent multiple joint pain in the past according to patient's recall.
Neuro-ophthalmologic examination showed impairment of the lateral gaze of the left eye, suggesting damage to left 6th cranial nerve. According to the Medical Research Council grades4), the muscle strength of the left upper and lower extremities is grade 3 and 4, respectively. An initial computed tomography (CT) of the brain showed no abnormalities. Diffuse axonal injury was suspected, and the patient was treated with dexamethasone (5 mg/day), leading to a gradual improvement in his muscle strength but diplopia was static. Although the CT of the brain on admission was normal, T2-weighted and fluid-attenuated inversion-recovery images on the MRI scan which was done in the next day showed abnormal high signal intensity with swelling of the right mesodiencephalic region (Fig. 1). Some kind of venulitis was suggested but there was no cerebral venous thrombosis.
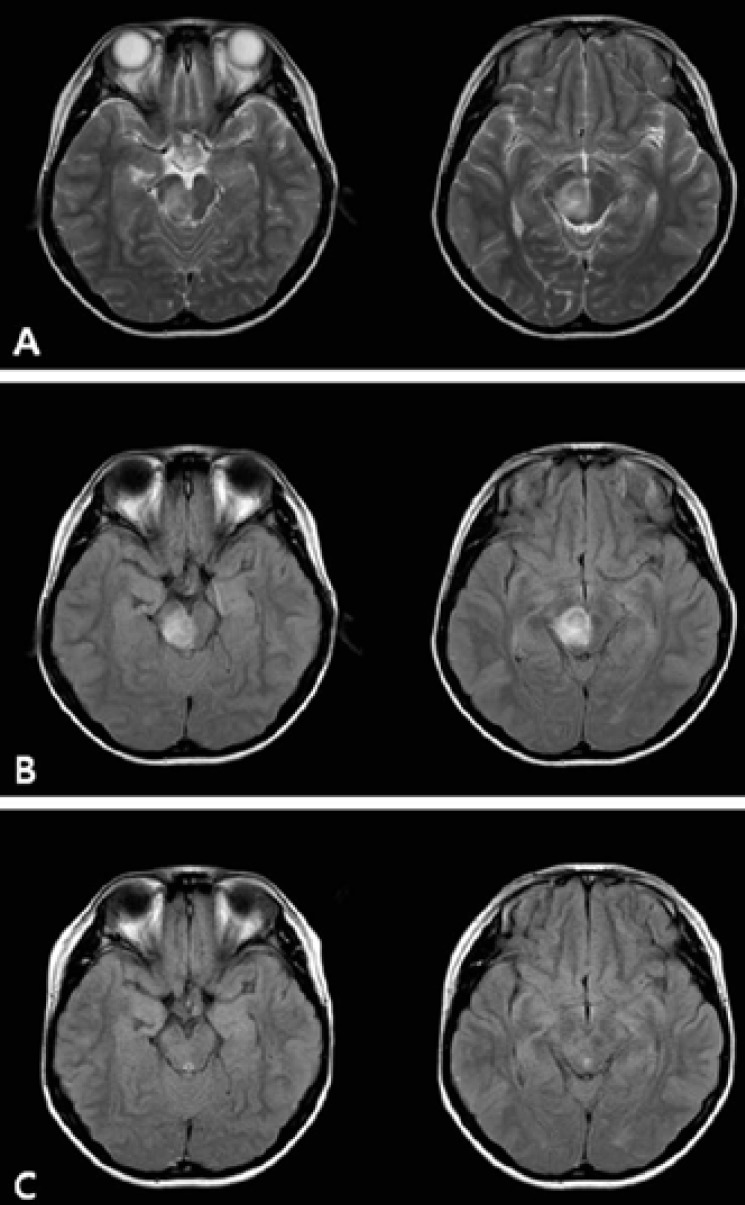
Brain magnetic resonance imaging study of the patient. T2-weighted axial image of the (A) hyperintensity of the right mesodiencephalic portion and (B) axial fluid attenuated inversion recovery image hyperintensity of the right mesodiencephalic portion obtained 1 day after admission. (C) The follow-up magnetic resonance imaging scan shows no evidence of abnormality after 2 weeks.
The results of white blood cell count, C-reactive protein and urinalysis were within normal limits. The laboratory studies for C3, C4, rheumatoid factor, anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-Ro/La antibodies and HLA B51 revealed all negative findings. We did not exam the cerebrospinal fluid (CSF) analysis because he had no signs of infection in the CNS such as neck stiffness, nausea, vomiting or fever. Despite a negative pathergy test and the absence of typical genital or ocular lesions, the diagnosis of NBD was suggested because of the history of recurrent oral aphthous ulcer, joint pain, and characteristic findings of the brain lesions in the MRI.
Subsequently, pulse methylprednisolone was initiated (500 mg/day for 3 days; 250 mg/day for 3 days; 125 mg/day for 3 days), followed by daily oral prednisolone at 1 mg/kg/day for 28 days. His neurologic status was gradually improved. After 9 days of pulse methylprednisolone therapy, the second MRI scan showed complete improvement of the brain lesion accompanied by an improvement of diplopia and left sided weakness (Fig. 1). During the following 2-month, diplopia or left sided weakness was not recurred.
Discussion
The diagnosis of BD in children is sometimes difficult because all specific diagnostic manifestations may not be present at the same time and long time intervals may be needed before the appearance of characteristic clinical features to make a definite diagnosis5). Since there is no laboratory diagnostic test, the diagnosis of BD depends on essentially clinical findings after the exclusion of other possible diseases. In this case, he had solid neurological manifestations and recurrent history of oral ulceration to be clinically considered NBD. The differential diagnoses include systemic lupus erythematosus (SLE), primary Sjögren syndrome, primary anti-phospholipid antibody syndrome, multiple sclerosis (MS), neurosarcoidosis, viral infections and systemic vasculitis6).
Typical autoantibodies suggesting a diagnosis of SLE, primary Sjögren syndrome and primary anti-phospholipid antibody syndrome were absent, and the patient did not fulfill the criteria for SLE or other collagen vascular diseases. The predominant motor system involvement and lack of sensory symptom is a characteristic feature of NBD but an unusual finding in MS. The lack of pulmonary abnormalities and the MRI findings also support the exclusion of neurosarcoidosis. The differentiation of MRI findings in NBD from those found in MS and neurosarcoidosis is based on predilection for different regions. Kocer et al.7) reported that the most commonly affected regions of the NBD were the mesodiencephalic area (46%), the pontobulbar region (40%), and the hypothalamic-thalamic region (23%). On the other hand, the lesions seen in MS affect predominantly the periventricular area and corpus callosum8). And there is predilection for hypothalamus, pituitary gland, leptomeninges, and cranial nerves in neurosarcoidosis9). The MRI image of our patient was compatible with the characteristic of adult NBD.
The patient had no symptoms of fever, neck stiffness or viral illness. And the vasculitis outside the brain was absent. Fujikawa and Suemitsu10) described the clinical characteristics of BD in children based on a retrospective study of patients with BD. They reported that entero-BD was more frequent in children than adults, while ocular complication and NBD were less frequent. The frequency of HLA-B51 was lower (14.4%) in children than adults. Four among 31 Japanese children with BD had developed NBD. The age of onset was variable (from 10 months to 13 years), and they had various type of NBD, such as transverse myelitis, meningitis, encephalitis, and convulsions.
Fujikawa and Suemitsu10) suggested periodontitis, recurrent tonsilitis and rubella were estimated to be inducing factors for the onset of disease. Our patient had a history of minor head trauma. Jones et al.11) suggested a causal relationship between CNS trauma and the onset of autoimmune pathology in genetically susceptible animals by explaining the overwhelming increase in the Th1:Th2 cytokines ratios in mice with traumatic injury. Hirohata et al.12) demonstrated that CNS inflammation play a critical role in the pathogenesis of progressive neuropychiatric manifestations of BD by the elevated CSF level of interleukin-6.
An international collaboratory study has also reported that patients who did not fulfill the international criteria of BD had significantly less frequencies of genital aphthosis, skin lesions hypersensitivity, or uveitis and more frequent neurologic manifestations13). In 33% of children who did not fulfill the international criteria of BD had neurologic symptoms, such as benign intracranial hypertension, hemiparesis, encephalopathy, brainstem syndrome, meningitis and cerebellar syndrome while 15% of children with BD had neurologic symptoms.
We described the clinical course of a 9-year-old patient who do not fulfill the diagnostic criteria for BD, but show the typical features of NBD. Hentati et al suggested terms 'probable neuro-Behçet' and 'possible neuro-Behçet' for such cases14). Akman-Demir et al.15) reported neurologic onset before any other symptom of BD was seen in 3% of the cases. Concomitant manifestation of neurologic symptoms with other symptoms of BD was seen in 7.5% of all patients.
NBD has a remarkable impact on the quality of life and needs to be treated as early as possible. However, because of the insidious nature of the disease, BD is hardly to be diagnosed until it is fully developed. Therefore, specific diagnostic criteria for childhood BD should be established through the consortium of the cases of BD. The brain MRI was indispensable for establishing diagnosis and follow-up.
In conclusion, we should consider the possibility of NBD as the cause of neurologic manifestations developed by minor head trauma in patients who have no alternative diagnosis. In addition we have to fully evaluate the evidence of BD if someone has typical features of NBD on the brain MRI with neurological abnormalities.
