Bone mineral density deficits in childhood cancer survivors: Pathophysiology, prevalence, screening, and management
Article information
Abstract
As chemotherapy and other sophisticated treatment strategies evolve and the number of survivors of long-term childhood cancer grows, the long-term complications of treatment and the cancer itself are becoming ever more important. One of the most important but often neglected complications is osteoporosis and increased risk of fracture during and after cancer treatment. Acquisition of optimal peak bone mass and strength during childhood and adolescence is critical to preventing osteoporosis later in life. However, most childhood cancer patients have multiple risk factors for bone mineral loss. Cancer itself, malnutrition, decreased physical activity during treatment, chemotherapeutic agents such as steroids, and radiotherapy cause bone mineral deficit. Furthermore, complications such as growth hormone deficiency and musculoskeletal deformity have negative effects on bone metabolism. Low bone mineral density is associated with fractures, skeletal deformity, pain, and substantial financial burden not only for childhood cancer survivors but also for public health care systems. Thus, it is important to monitor bone health in these patients and minimize their risk of developing osteoporosis and fragility fractures later in life.
Introduction
Overall long-term survival of childhood cancer patients is increasing and is estimated to be 74% to 80% because of early detection, evolution of chemotherapy, improved surgical techniques, refined radiation therapy, and hematopoietic stem cell transplantation (HSCT)1,2). It is estimated that 1 of every 640 young adults in the USA is a childhood cancer survivor (CCS)3). However, long-term survivors of childhood cancer (LTSCC) face increased risks of various health problems resulting from treatment or the cancer itself. These include secondary cancers, cardiac dysfunction, infertility, growth failure, endocrine deficiencies, renal damage, pulmonary toxicity, hearing loss, neuropsychiatric problems, obesity, and musculoskeletal abnormalities4,5). Among these, osteoporosis is of great importance because it can lead to fractures, pain, deformity, gait abnormality, and substantial financial burden6-8).
Osteoporosis was previously considered a disease of the elderly but is now considered to have a pediatric origin6). Individuals who fail to achieve optimal peak bone mass (PBM) and strength during childhood and adolescence are more likely to develop osteoporosis later in life9). LTSCC fail to achieve optimal PBM because of the cancer itself, malnutrition during chemotherapy, and decreased physical activity. The onset of most childhood cancers is in the second decade of life, when nearly 43% to 66% of maximal adult bone mineral density (BMD) is gained10). Thus, LTSCC have a high prevalence of osteoporosis or low BMD, even in early adulthood11-22). Radiotherapy, chemotherapy, and hormone deficiencies are frequently cited as risk factors for osteoporosis and fractures in LTSCC13,18,23,24).
In this review, we address the pathophysiology, prevalence, screening, and management of osteoporosis in CCSs. The objective is to raise awareness among pediatricians of the risk of osteoporosis in CCS and the 2008 Children's Oncology Group (COG) long-term follow-up (LTFU) guidelines for bone mineral deficits13).
Pathophysiology of BMD deficits in CCSs
The mechanism causing BMD deficits in CCS is multifactorial. It depends on cancer type, therapeutic modalities such as chemotherapeutic agents and radiation, and the occurrence of endocrine complications like growth hormone deficiency (GHD) and hypogonadism. In addition, other factors such as age at cancer diagnosis, sex, genetic predisposition, nutrition, and lifestyle affect BMD.
Cancer itself causes BMD deficits. Ten to twenty percent of children with acute lymphoblastic leukemia (ALL) exhibit reduced BMD at diagnosis25,26). Leukemic infiltration of the bone and various factors secreted by leukemic cells, such as parathyroid hormone (PTH) and PTH-related peptide, were suggested as causes of BMD deficit25). Some osteosarcoma patients have had BMD deficit in the affected limb, which initially presented with bone pain26).
Chemotherapeutic agents such as corticosteroids, methotrexate, and alkylating agent are well known to cause BMD deficits directly or indirectly. Corticosteroids inhibit new bone formation by decreasing osteoblastic activity, including osteocalcin production, and by directly increasing osteoclastic bone resorption23). Corticosteroids also inhibit 1α-hydroxylation of vitamin D and result in impaired intestinal absorption of calcium and reduced muscle strength. ALL patients administered a total corticosteroid dose greater than 9 g/m2 are more likely to develop reduced BMD that does not recover to within normal range after treatment27). High-dose prednisone (≥20 g/m2) is a risk factor for osteopenia in patients with malignant lymphoma15). Methotrexate also suppresses osteoblast activity and stimulates osteoclast recruitment, resulting in decreased bone formation and increased bone resorption28). Higher cumulative doses of methotrexate have been associated with a greater incidence of osteopenia23). Total doses of more than 4 g/m2 were associated with a high risk of osteopenia and failure to recover to a normal BMD after completion of therapy16,27). Alkylating agents such as cyclophosphamide and ifosfamide cause hypogonadism and result in decreased BMD. Estrogens play a crucial role in achieving and maintaining PBM by preventing bone resorption and stimulating growth factors necessary for bone growth29). Androgens seem to have an important role in periosteal apposition, adding to the biochemical strength of the bone29). Ifosfamide also induces Fanconi syndrome, which can result in hypophosphatemia and severe metabolic bone disease. Other agents such as vincristine, daunorubicin, etoposide, and asparaginase reduce type I collagen synthesis30). Vincristine also induces neuropathy, which leads to poor balance and increases the risk of falls.
Radiation is the most important risk factor for osteoporosis, especially in brain tumor survivors24). Radiation might directly affect BMD by damaging the bone marrow stroma31), but the principal cause is assumed to be deficiencies of hormones such as growth hormone (GH) and sex steroid7,17). Cranial and total body irradiation (TBI) can lead to GHD and central hypogonadism, both of which are associated with BMD deficits32). Children who received cranial irradiation at a dose of 24 Gy had reduced spontaneous and pulsatile GH secretion, while nonirradiated children had normal secretion13). Fractionated TBI doses of >12 Gy for transplant conditioning may also result in GHD13,18). Radiation-induced fractures in survivors treated with radiation doses of ≥40 Gy have been described33).
Endocrine sequelae such as GHD and hypogonadism also cause BMD deficits in CCS. GH is important to acquiring adequate PBM and to maintaining bone mass34). Not only radiation but also chemotherapy can cause GHD. Roman et al.35) reported that 44% of a cohort of 25 children who had received chemotherapy and surgery for malignant solid tumors developed impaired GH secretion. GHD is related to low BMD in both adults and children14,34,36). Baroncelli et al.36) reported that lumbar BMD declined rapidly after discontinuation of GH in patients with GHD, compared with controls. We also reported that GH replacement was associated with increase in BMD in patients with intracranial germ cell tumors14).
Hypogonadism is often caused by alkylating agents and radiation. Gonadotropin deficiency occurs with high-dose radiation (≥40 Gy) to the neuroendocrine axis13). Peripheral hypogonadism occurs at much lower radiation doses. Ovarian dysfunction and premature menopause are associated with radiation doses of ≥10 Gy to the ovaries in prepubertal females and ≥5 Gy in pubertal females13,18). In males, higher doses (≥20 Gy) are required to cause Leydig cell dysfunction with associated androgen insufficiency13,18).
HSCT is well known to cause BMD deficits. Adult transplantation studies reported that a 2% to 10% loss of BMD significantly increased the risk of fractures37). Childhood leukemia survivors with HSCT had gonadal deficiency and significantly lower femoral BMD as adults38). Patients undergoing HSCT often receive multiple agents associated with altered bone metabolism, including methotrexate, steroids, TBI, and high-dose alkylating agents, as part of their treatment19). In addition, altered kidney, liver, and bowel functions might result in reduced absorption and abnormal metabolism of calcium and vitamin D39). Finally, the HSCT procedure itself has been reported to cause severe and persistent quantitative and qualitative impairment of osteoblastic precursors within the stromal stem cell compartment38).
Some CCS may fail to achieve optimal PBM. To acquire adequate PBM, nutrition and physical activity are essential9). However, most pediatric cancer patients do not regularly consume the recommended daily allowances of nutrients, including during cancer treatment. Such patients have been found to have altered metabolism of calcium, 1,25-dihydroxyvitamin D, and magnesium during prolonged periods of hospitalization and immobilization40,41). During and after treatment, they were less involved in physical activities than healthy controls12,42). Pain associated with osteopenia significantly limited physical activity in brain tumor survivors12). Reduced exercise capacity was related to decreased bone mineralization in survivors of ALL42). CCS seemed to have a higher prevalence of osteoporosis and fractures in males than in females14,42-44). The longer period of pubertal development and partial hypogonadism in boys might contribute to this difference between sexes14,43).
Prevalence of BMD deficits in CCSs
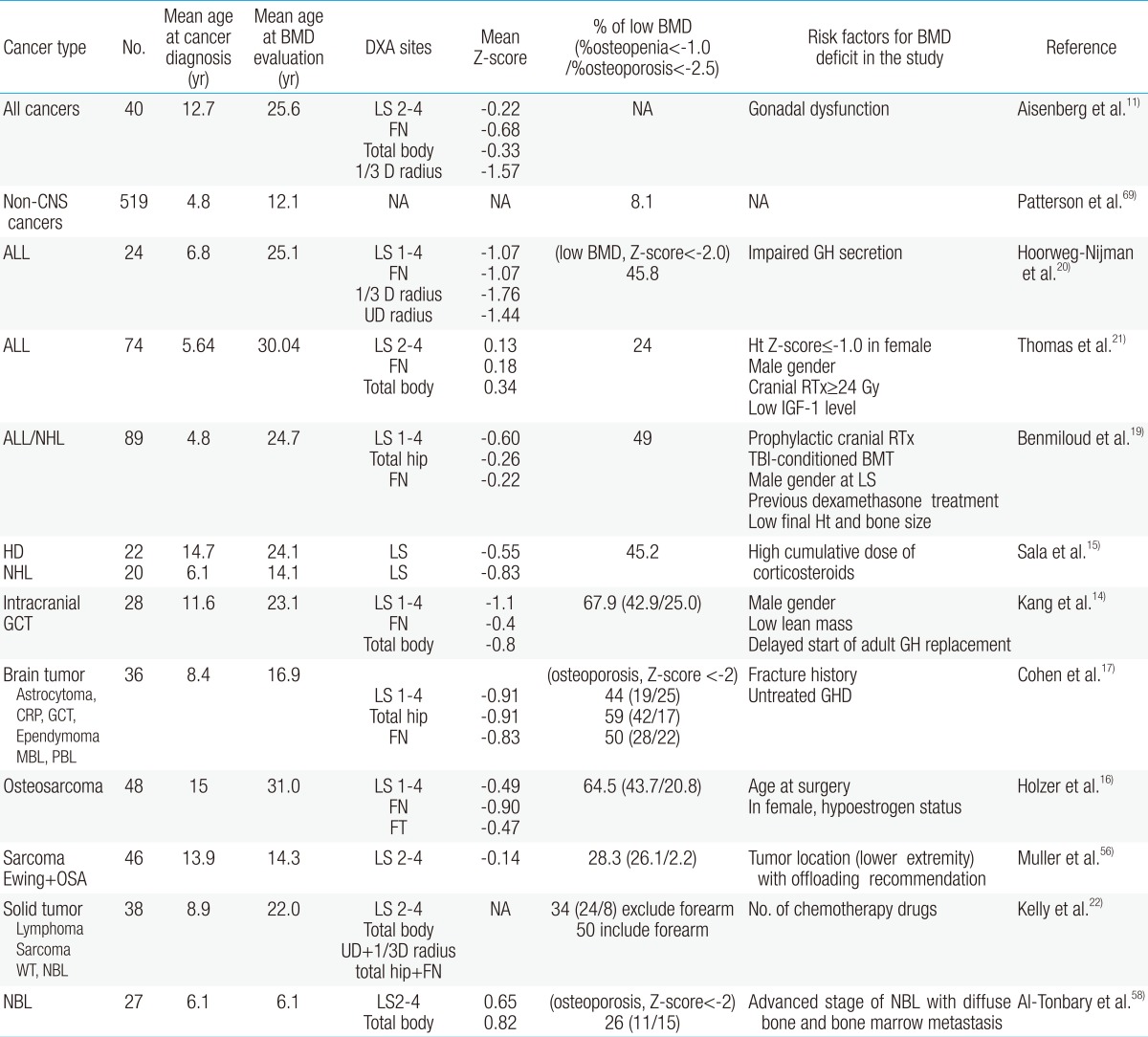
The exact prevalence of osteoporosis and osteopenia in CCS is difficult to show, although published studies have identified reduced BMD as a common finding in young adulthood. Most reports include small numbers of subjects, diverse malignant diseases, heterogeneity of therapeutic modalities, various age groups, different sites of BMD measurement, and different definitions of osteoporosis (Table 1). The Childhood Cancer Survivor Study reported results of a questionnaire survey of more than 1,600 brain tumor survivors, only 29 of whom had osteoporosis45). However, other studies reported osteoporosis or osteopenia in almost half of the CCSs11-22,46). Therefore, the true prevalence of bone mineral loss could be underestimated according to methods of BMD evaluation in CCS.
The terminology and criteria of low BMD are diverse and mixed in the literature, although "low bone density for chronologic age" is the preferred wording in CCS studies. World Health Organization definitions of osteopenia and osteoporosis are based on T-scores, used to compare patients' BMD with the maximum BMD of young adults, and applied only to postmenopausal women. The International Society for Clinical Densitometry recommended use of "low bone density for chronologic age" when the BMD Z-score is lower than -2.0 for males and females younger than 50 years47). Wasilewski-Masker et al.32) and Sala et al.15) suggested another criterion: Z-score less than -1.0 was defined as low BMD, and Z-score less than -2.5 as significantly low BMD. In our previous study, Z-scores of <-2.0 indicated osteoporosis, and Z-scores ≥-2.0 and <-1.0 indicated osteopenia in subjects younger than 20 years14). For convenience, we used the terms "osteopenia" and "osteoporosis" when searching the literature. The prevalence rates of osteoporosis and osteopenia in CCS are summarized in Table 1.
1. ALL
At diagnosis, 10% to 20% of children with ALL exhibited reduced BMD at the lumbar spine7,48). During therapy, additional bone mineral loss occurred, especially in early periods of intensified treatment, and approximately 50% of children had a reduction in BMD. Halton et al.48) reported that fractures occurred in 39% of children during treatment. Reports of results from after completion of therapy have been conflicting. Kaste et al.43) reported that ALL patients had a median BMD Z-score of -0.78 standard daviation (SD), and 21% had abnormally low BMD, compared to 5% in normal populations after 15.9 years. Hoorweg-Nijman et al.20) also concluded that bone development in patients cured of ALL is disturbed, resulting in a significantly reduced BMD. In a 24-year follow-up study, low BMD was found to be more prevalent (24%) than expected based on population normative data, specifically in men21). However, some reported normal BMD after cessation of ALL treatment49-51). Brennan et al.52) reported that adult survivors of ALL whose treatment included cranial irradiation had reduced BMD, while those without cranial irradiation showed normal bone mineral apparent density (BMAD)53).
2. Lymphoma
Osteopenia (Z-score<-1.00) was found in 41% (9/22) of patients with Hodgkin's disease and 50% (10/20) of patients with non-Hodgkin's lymphoma at the lumbar spine after 24.1 and 14.1 years of follow-up, respectively15). The use of mechlorethamine, oncovin, procarbazine, and prednisone led to decreased height and increased body mass index in men and decreased total body BMD in women54). High cumulative dose of corticosteroids, especially, was a risk factor for osteopenia15).
3. Brain tumors
Osteoporosis is a common sequel of therapy in children with brain tumors. We previously reported that 25.0% of patients with germ cell tumors had osteoporosis and 42.9% had osteopenia at 10.9-year follow-up14). The prevalence of osteoporosis (bone mineral contents Z-score<-2.5) in the entire body was more than 40% in the irradiated group and 0% in the nonirradiated group of patients with posterior fossa tumors or optic glioma46). Barr et al.12) also reported that BMD decreased at the lumbar spine (mean Z-score, -1.05) and femoral neck (mean Z-score, -0.84), with an overall osteopenia prevalence of 47.3%, in brain tumor survivors. Previous reports have showed that survivors of various childhood brain tumors are susceptible to development of fractures and early osteoporosis, especially in association with the risk factor GHD17,45).
4. Other solid tumors
Low BMD in has been reported in survivors of various other solid tumors22). Osteopenia or osteoporosis was reported in 28.3% to 65% of survivors of osteogenic sarcoma or Ewing's sarcoma16,55,56). Holzer et al.16). reported that 65% of osteosarcoma patients had BMD deficits at the age of 31±4.2 years, and the mean duration of follow-up from diagnosis was 16±2.2 years. We also found that 47.5% of long-term survivors of osteosarcoma had osteoporosis and 30.0% had osteopenia at the age of 22.0±5.1 years (unpublished data). A high incidence of osteopenia (26%) was found in survivors of Wilms' tumor57) and neuroblastoma58). Low bone mass may occur in children at initial diagnosis of neuroblastoma and before the start of chemotherapy or radiotherapy, especially if the disease is at an advanced stage with diffuse bone and bone marrow metastases58).
5. HSCT
HSCT recipients had reduced BMD at multiple skeletal sites and BMAD in the spine when compared with controls59). In a prospective study, BMD of the spine and hip gradually decreased over 12 to 14 months after allogeneic HSCT60). Kaste et al.61) reported that after at a median follow-up of 5.1 years, allogeneic HSCT recipients had a median BMD Z-score of -0.89, and 14.6% had osteopenia. In a 14-year follow-up study, acute leukemia patients in the HSCT group had a significantly reduced femoral neck BMD (mean BMD Z-score, -0.49) compared with patients who did not receive a transplant, and 7.4% of them had a history of fracture. Children who were younger at the time of transplantation had a significantly higher risk of low spinal BMD during adulthood38). Bone loss seemed to be worse after allogeneic HSCT than after autologous HSCT62), probably because of a prolonged and greater cytokine release after transplantation and because use of immunosuppressive agents is more important after allogeneic transplantation.
Screening and management
The high prevalence of osteoporosis, even in early adulthood, in CCS suggests that specific diagnosis and treatment may be beneficial, although there is still no consensus on the treatment of osteoporosis in the young. The 2008 COG LTFU guidelines recommended a baseline evaluation of BMD by dual-energy X-ray absorptiometry or quantitative computed tomography at entry into LTFU for patients exposed to methotrexate, corticosteroids, and HSCT and who have medical conditions such as GHD, hypogonadism, delayed puberty, or hyperthyroidism13). Some experts even insist that children with brain cancer should be evaluated for BMD immediately, because most cancer treatment protocols are risk factors for bone mineral deficit63). BMD should be evaluated with robust normative data; fortunately, we have normative data for Korean children and adolescents10).
The management algorithm for osteoporosis in CCS is shown in Fig. 1. All CCS should undergo prophylaxis for bone loss and fractures (Table 2). Repeat measurements are not required for patients with normal baseline BMD results (BMD Z-score>-1). The COG LTFU guidelines recommend 800 to 1,500 mg of calcium and 200 IU vitamin D daily intake, through diet or supplementation13). However, pediatricians should be aware of the fact that most Korean children and adolescents are not receiving the recommended dietary intake of calcium and vitamin D64). Recently, the U.S. Institute of Medicine issued a new clinical guideline on vitamin D65). According to this guideline, 600 IU vitamin D is needed to maintain 25-hydroxyvitamin D at more than 20 ng/mL. Considering the fact that corticosteroids reduce intestinal calcium absorption and increase calcium excretion, adequate intake of calcium and vitamin D is an important prevention strategy. Up to 1,500 to 2,000 IU vitamin D is needed in cases of vitamin D deficiency.

Algorithm to prevent osteoporosis in childhood cancer survivors. LTFU, long-term follow-up; GHD, growth hormone deficiency; DXA, dual energy X-ray absorptiometry; BMD, bone mineral density.
If BMD Z-score is between -1.0 and -2.0, regular follow-up is required. Treatment of BMD deficits in children includes increasing weight-bearing exercise as tolerated, optimizing nutritional intake of calcium and vitamin D, nutrient supplementation if dietary intake is insufficient, and treatment of conditions that may exacerbate BMD deficit, such as hypogonadism or GHD. Running or jumping has been proven to positively affect BMD8). Supplementation with vitamin D and increased calcium intake have been proven to enhance PBM in healthy children. Counseling survivors about avoiding smoking, alcohol, and caffeine is also important, as all of these can exacerbate BMD deficits66).
If BMD Z-score is less than -2.0, recurrent fractures, latent medical risk factors for decreased BMD, and hormonal risk factors such as hypogonadism or GHD should be considered. Endocrinology consultation may be advisable. Pediatric endocrinologists should thoroughly evaluate BMD and osteoporosis risk factors in cancer survivors. They also should consider treatment options such as bisphosphonates, although bisphosphonates are currently reserved for patients with recurrent fractures and clinical trial participants. Only a few publications on the use of bisphosphonates in pediatric cancer patients are available, but reports of bisphosphonate treatment benefit in cancer patients are limited67,68). Pamidronate caused rapid pain relief, gradual improvement of BMD, and improved motor function68). These are promising results, but long-term controlled studies are needed to assess the risks and benefits of bisphosphonates in children with osteoporosis.
Conclusions
Survivors of childhood and adolescent cancers are at risk for osteoporosis (or low bone mass for chronologic age) during and after completion of therapy. Treatment with corticosteroids, methotrexate, or HSCT is associated with an increased risk of BMD deficits. Radiation and alkylating chemotherapy increase the risk of endocrinopathies that predispose patients to reduced BMD. Genetic predisposition and lifestyle may also contribute to BMD deficits. Pediatricians should be aware that CCS are at risk for osteoporosis and require appropriate early intervention.