Enterovirus infection in Korean children and anti-enteroviral potential candidate agents
Article information
Abstract
Although most enterovirus infections are not serious enough to be life threatening, several enteroviruses such as enterovirus 71 are responsible for severe, potentially life-threatening disease. The epidemic patterns of enteroviruses occur regularly during the year, but they may change due to environmental shifts induced by climate change due to global warming. Therefore, enterovirus epidemiological studies should be performed continuously as a basis for anti-viral studies. A great number of synthesized antiviral compounds that work against enteroviruses have been developed but only a few have demonstrated effectiveness in vivo. No proven effective antiviral agents are available for enterovirus disease therapy. The development of a new antiviral drug is a difficult task due to poor selective toxicity and cost. To overcome these limitations, one approach is to accelerate the availability of other existing antiviral drugs approved for antiviral effect against enteroviruses, and the other way is to screen traditional medicinal plants.
Introduction
The clinical manifestations of human enterovirus (HEV) infections vary, ranging from asymptomatic, mild respiratory illness, and aseptic meningitis to serious symptoms such as myocarditis, encephalitis, acute flaccid paralysis, and severe neonatal sepsis-like disease1-3).
Fatal enterovirus (EV)71 infections are frequently reported4-6). EV71-infected children can develop severe neurological complications, which can lead to rapid clinical deterioration and even death7,8). Additionally, some reports have suggested that enteroviral diseases may be expanded to other serious human ailments such as autoimmunity, type 1 diabetes9), Sjögren's syndrome10), various neurological disorders11), and some tumors12).
The risks of emerging diseases associated with HEV infections have increased; however, there are no proven specific therapeutic antiviral agents or vaccines that can prevent HEV diseases. The only mainstay of treatment is supportive care to control fever and pain, thus, new and more effective antiviral agents for HEV infection are needed.
We describe HEV infections, including epidemiology in Korean children, and introduce current antiviral agent studies, although no proven antiviral drugs are available to date.
HEVs
1. Classification
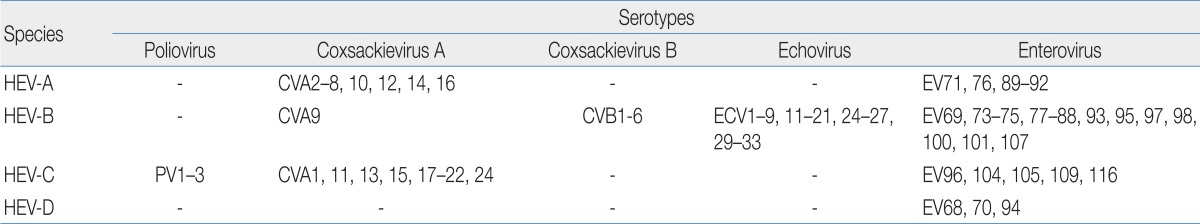
The HEV genus belongs to the Picornaviridae family. HEVs were originally divided into five subgroups, including polioviruses, group A coxsackieviruses (CVA), group B coxsackeviruses (CVB), echoviruses (ECV), and "newer" EVs. This genus, with the exception of polioviruses, comprises over 90 immunologically distinct serotypes that cause human infections. These viruses are also classified genetically into HEV-A to HEV-D, and shown in Table 113-16).
2. Pathogenesis
EV infections are acquired by ingestion or respiratory droplet that is shed in the feces or upper respiratory tract. Initial viral replication occurs in the mucosal tissues of the intestine and nasopharynx. Initial replication in the pharynx and intestine is followed within days by multiplication in lymphoid tissues such as the tonsils, Peyer's patches, and regional lymph nodes. A primary, transient viremia (minor viremia) results in a spread to distant parts of the reticuloendothelial system, including the liver, spleen, bone marrow, and distant lymph nodes.
Further replication in theses organs leads to a sustained "major" viremia with dissemination of the virus to target organs such as the central nervous system, heart, and skin, resulting in a clinical infection. Tropism to target organs is determined, in part, by the infecting serotype16,17).
3. Clinical manifestations
Although most infections in children subside mildly or asymptomatically, HEVs can also be responsible for severe, potentially life-threatening disease18).
HEVs can cause asymptomatic infections, nonspecific febrile illness, hand-foot-and-mouth disease, herpangina, and acute hemorrhagic conjunctivitis. HEVs can also cause respiratory diseases such as an exacerbation of asthma, apnea, respiratory distress, pneumonia, otitis media, bronchiolitis, croup, parotitis, pharyngotonsillitis, and pleurodynia. HEVs are involved in myocarditis and pericarditis as well as gastrointestinal diseases, such as emesis, abdominal pain, diarrhea, hematochezia, pneumatosis intestinalis, necrotizing enterocolitis, hepatitis, and pancreatitis. HEVs cause genitourinary diseases, such as orchitis, epididymitis, nephritis, and immunoglobulin A nephropathy and neurological manifestations including aseptic meningitis, encephalitis, poliomyelitis-like acute flaccid paralysis, brainstem encephalitis, Guillain-Barré syndrome, transverse myelitis, cerebellar ataxia, opsoclonus-myoclonus syndrome, as well as benign intracranial hypertension. HEVs are involved in cutaneous manifestations such as, exanthem, petechial rashes, purpuric rashes and papular acrodermatitis. HEVs have been associated with myositis, arthritis and neonatal infections.
HEV infections may initiate an inflammatory insult to pancreatic islet cells and thereby contribute to the development of type 1 insulin-dependent diabetes mellitus16,19).
4. Epidemiology of HEV infection
HEV infections are common in children particularly those <1 year of age20,21). Various HEV serotypes are spreading globally by temporal and regional distribution. Epidemics of HEV typically peak during the summer and fall, and predominant serotypes are often associated with a single outbreak22). The predominant serotypes vary from year to year, and HEV epidemics in Korea may be affected by environmental shifts induced by climate change due to global warming21,23). Therefore, systemic long-term epidemiological studies should be conducted to prevent the spread of HEVs and to identify proper anti-viral therapies.
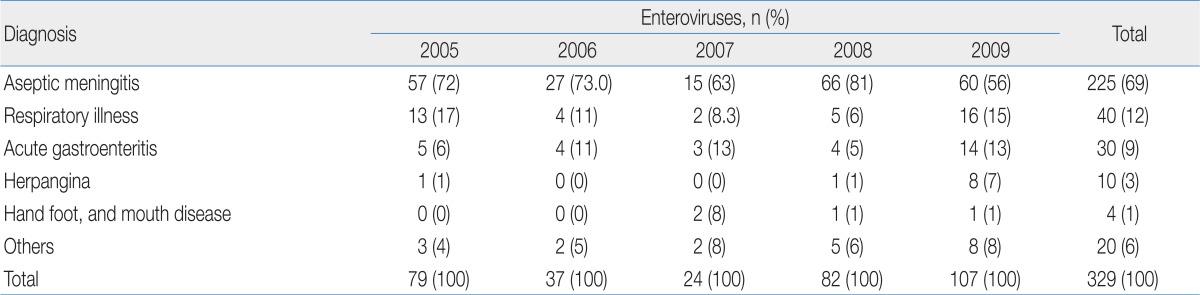
HEVs are the leading cause of aseptic meningitis, which is the most frequent central nervous system infection worldwide24,25). The isolation of HEVs often focuses on a single outbreak with aseptic meningitis. Aseptic meningitis in Korea was the most common manifestation in children with enteroviral infection22), the Chungnam data that is shown in Table 2 show similar results. Followed by aseptic meningitis, HEVs are isolated from patients with respiratory illness, acute gastroenteritis, herpangina, and hand foot mouth disease (HFMD) (Table 2)21,23). No disease is uniquely associated with any specific serotype, although certain manifestations are preferentially associated with specific serotypes26).
HEVs infect millions of people worldwide each year, mostly children and young adults, and different HEV serotypes are spreading globally each year. The prevalent HEV serotypes vary yearly among ECV6, 9, 18, and 30, but CVB1 is the type most frequently isolated in Europe and the USA in recent years27,28).
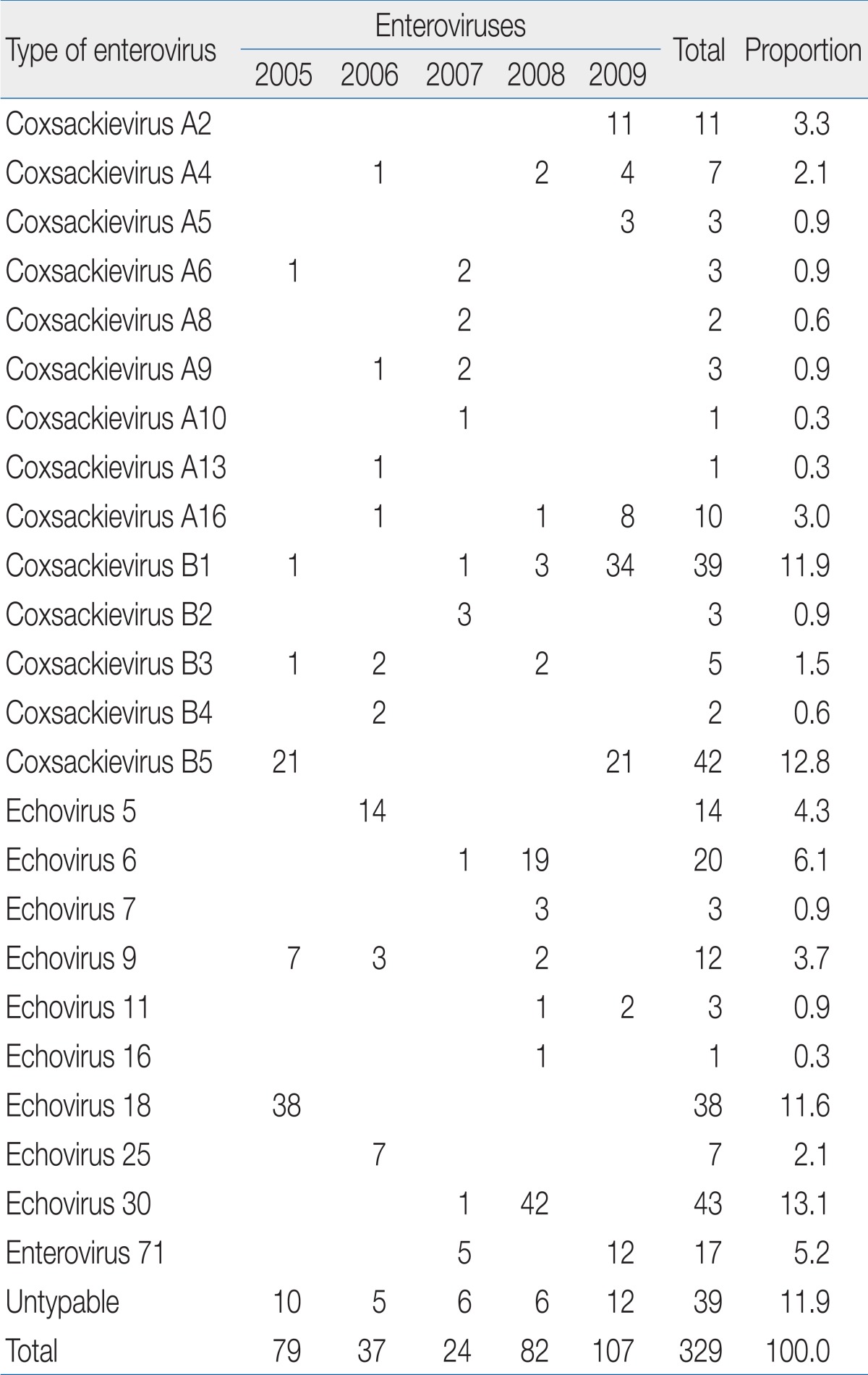
Since 1993, when nationwide surveillance began in Korea, there have been reports of summer outbreaks of various HEV types, such as ECV6, 9, 13, 30, and CVA2429). The recent epidemic of HEV serotypes in Chungnam, Korea included EV71, ECV5, 6, 18, 30, and CVB1 and 5 (Table 3) and reflected the national trend21,23). In a previous study, EV71 was detected in 5 cases among a total 24 enterovirus cases (20%) in 2007 and 12 cases (11%) among 107 cases in 2009. There were no identified cases of EV 71 in 2005, 2006 and 2008 (Table 3). Severe EV 71-induced HFMD with neurological complications was frequently reported in Korea nowadays26,30).
In individuals <1 year of age, the number of tested samples and HEV-positive cases were high in Chungnam, Korea (Table 4)21,23). Typically, HEV-positive cases are widely distributed throughout the year in tropical and semitropical regions, prominent in the summer and fall, but are far less detected in the winter and spring. The temporal pattern of HEV epidemics in Chungnam, Korea represents a remarkable seasonality, with cases occurring from June to August (Fig. 1)21,23). The ratio of male to female HEV-positive patients was approximately 1.6:1 in the Chungnam area.
Generally, serotypes of HEVs epidemic were different each year, and epidemic patterns may change due to environmental changes31,32). HEVs have a high mutation rate due to the absence of proofreading activity during genome replication. Additionally, about a 20% genetic difference occurred between prototype strains and the current widespread strains, and the difference was at the HEV cleavage site31,32). Thus, the development and screening of antiviral drugs against HEV should focus on the current epidemic strains.
Antiviral agents against HEVs
No HEV-specific antiviral drugs have been available for clinical use until recently. A great number of synthesized antiviral compounds against HEVs in vitro have been described but only a few of them have shown effectiveness in vivo, and none have been approved for clinical use33,34).
1. Current antiviral drugs
The current antiviral drug armamentarium comprises approximately 40 compounds that have been formally licensed for chemotherapy in humans with viral infection. Most are used to treat infections caused by human immunodeficiency virus, hepatitis B virus, herpesviruses, varicella-zoster virus, cytomegalovirus, influenza virus (IFN), respiratory syncytial virus, and hepatitis C virus. At present, there is no specific antiviral drug to treat or prevent diseases caused by HEVs35).
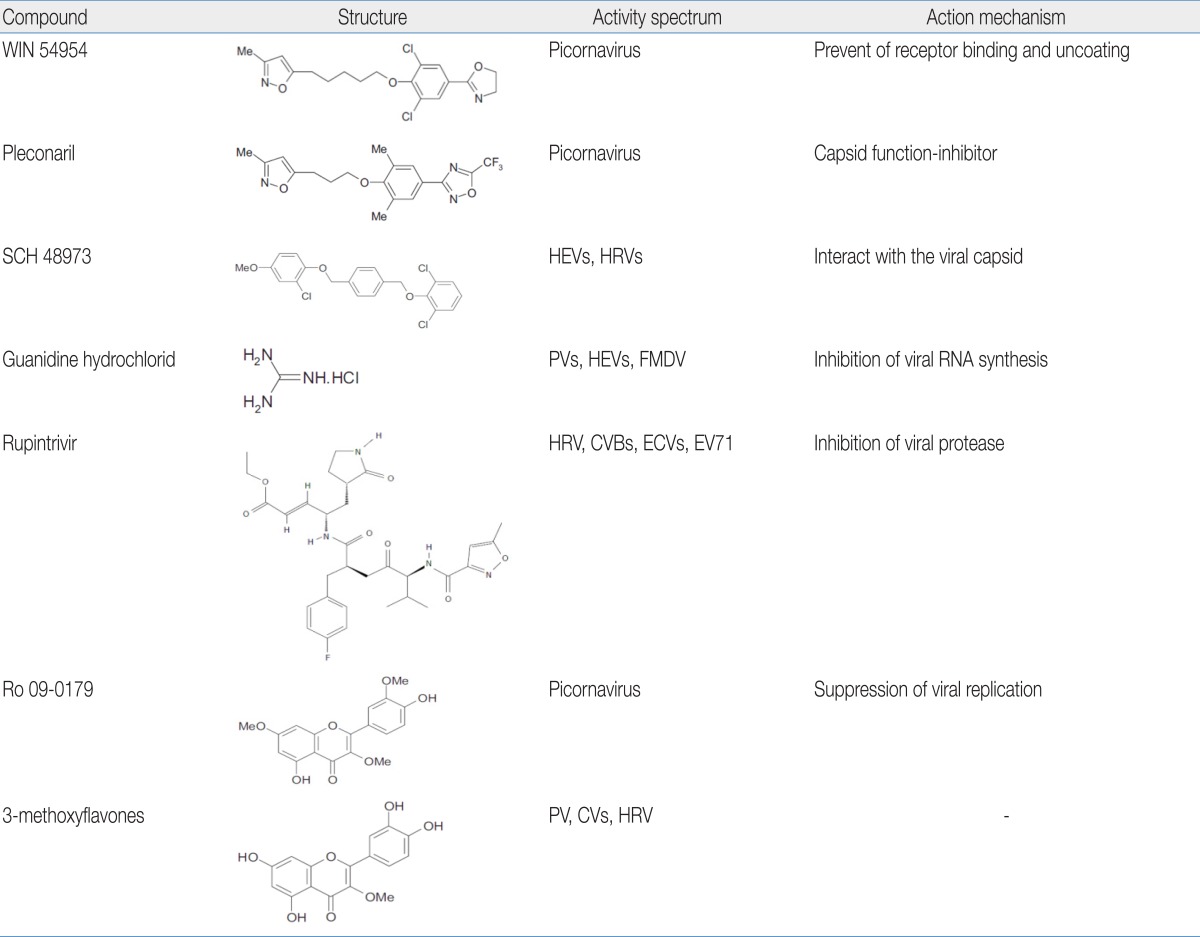
1) WIN series
The commonly known "WIN" compounds developed by Sterling-Winthrop represent the first demonstration of oral efficacy of an anti-HEV agent. These compounds moderately prevent virion uncoating activity against HEVs both in vitro and in vivo and have advanced to clinical trials 18,36,37). WIN 54954 significantly reduces the number and severity of colds induced by CVA21 and also significantly reduces nasal mucous discharge, respiratory and systemic symptoms, and viral titers18,38). But, WIN 54954 was not further developed for clinical use because of adverse flushing and rash reactions, possibly related to concomitant alcohol ingestion by study volunteers.
Pleconaril (WIN 61893) is the first of a new generation of metabolically stable capsid function inhibitors. In a mouse model of multi-organ system infection following intracranial inoculation of HEVs, pleconaril reduced viral titers in all affected organs and prevented death in animals. The oral bioavailability in humans and other animals approaches 70%18,39). Despite promising initial results, the drug was recently found to have no detectable clinical or virological benefit in infants with EV meningitis compared to that of a placebo18,40).
2) SCH series
The SCH series developed by Schering-Plough inhibits plaque formation of several HEVs and protects mice infected with either CVB3 or ECV9 from mortality. These molecules act at an early stage in the viral replication cycle, and specifically interact with the viral capsid. However, the compounds do not prevent the attachment of the virus to the host cell, but rather exert their activity after the initial stage of viral uncoating18,41).
3) Guanidine hydrochloride
Guanidine hydrochloride inhibits the replication of polioviruses, several CVs, and ECVs18,42). Early studies using crude RNA replication complexes in vitro indicated that guanidine inhibited the initiation of viral RNA synthesis without inhibiting the elongation or release of completed positive-strand RNA molecules. A later study confirmed that the compound inhibits a 2C function that is required to initiate negative but not positive-strand RNA synthesis during RNA elongation18,43).
4) Rupintrivir
Rupintrivir, developed by Pfizer, is a synthetic compound that is effective against all serotypes of human rhinovirus (HRV) and several HEV serotypes (CVB2 and 5, ECV6 and 9, and EV71) with low toxicity. This compound is highly specific for picornaviral protease and but is not active against cellular serine and cysteine proteases but has excellent irreversible inhibition capacity against protease44). Rupintrivir has demonstrated moderate antiviral and clinical efficacy in Phase II clinical trials. But, further development was stopped because of a lack of efficacy in natural infection studies. The structures of the above listed compounds are shown in Table 518,44).
2. Medicinal plant-based antiviral agents
An easy way to overcome the limitation of antiviral drugs is the screening of traditional medicinal plants. Medicinal plants, for their ability to have multiple targets, minor side effects, low potential to cause resistance, and low cost may be suitable alternative sources of antiviral agents. The screening of medicinal plants as a possible source of antiviral agents has led to the discovery of potent inhibitors of in vitro viral growth and the use of the ethnopharmacological approach which enhances the probability of identifying new bioactive plant compounds45,46). The structures of the below listed compounds are also shown in Table 518). Oseltamivir, was marketed under the trade name Tamiflu (Roche, Basel, Switzerland), is an IFN antiviral drug that originated from shikimic acid isolated from Chinese star anise, an ancient cooking spice; this herb is also used in traditional Chinese medicine.
1) Ro 09-0179
Ro 09-0179, which was isolated from a Chinese medicinal herb, exhibits in vitro antiviral activity against a broad range of picornaviruses. This compound has been suggested to interfere with some viral replication processes that occur between viral uncoating and the initiation of viral RNA synthesis18,47).
2) 3-Methoxyflavones
From a series of naturally occurring 3-methoxyflavones isolated from plant extracts of Euphorbia grantii, 3-methylquercetin proved to be the most promising agent with in vitro antiviral activity against poliovirus, CVs, and HRVs. This compound protects mice against a lethal CVB4 infection48).
3) Chinese medicinal plants
Among Chinese medicinal plants, Sargentodoxa cuneata (Oliv.) Rehd. Et Wils., Sophora tonkinensis Gapnep., Paeonia veitchii Lynch, Spatholobus suberectus Dunn, and Cyrtomium fortunei J. sm. have antiviral activity against CVB3 and 5 as well as ECV9 and 29 in an in vitro test. However, it is unclear what these results signify for in vivo effectiveness. The results obtained in this preliminary screening justify continuing with the purification of crude extracts and isolating active compounds to assess their potential as antiviral drugs and/or finding new lead molecules49). Although several hundreds of plants that have potential as novel antiviral agents have been studied, there still exists innumerable potentially useful medicinal plants waiting to be evaluated and exploited for therapeutic applications against genetically and functionally diverse virus families45,46).
3. Strategy of searching for an antiviral agent
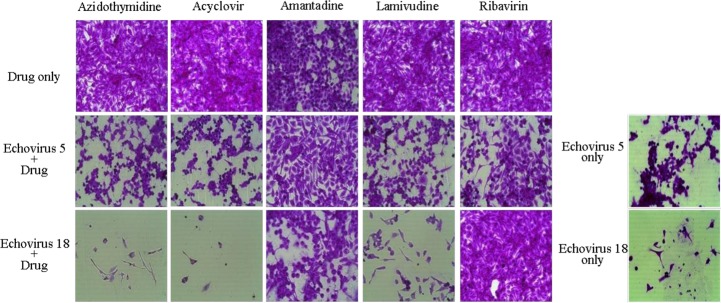
The development of a new antiviral drug is a difficult task considering the poorly selective toxicity and cost. To screen traditional medicinal plants is one way to overcome these problems. Another way to accelerate the availability of existing antiviral drugs is to approve them for an HEV antiviral effect. For example, amantadine, an M2 channel inhibitor for IFN and ribavirin that showed antiviral activity against ECV5 and 18 as well as EV71 in an in vitro test (Fig. 2)31,32,50).
Conclusions
This paper provides an overview of HEV infections, including the epidemiology of HEV infections in Korean children. HEVs cover a large genus of pathogens and can also be responsible for severe, potentially life-threatening diseases. However, until recently no HEV-specific antiviral drugs were available for clinical use. A great number of synthesized antiviral compounds directed against HEVs have been described but none have been approved for clinical use yet. Therefore, anti-enteroviral studies should be variously performed such as screening for traditional medicinal plants, the reposition of existing antiviral drugs as well as the development of new synthesized compounds in a search for antiviral agents against HEVs in the future. In addition to the acceleration of proper identification of antiviral treatments, systemic long-term epidemiological studies for HEV infections should be carried out.
Acknowledgment
This study was supported by the Soonchunhyang University Research Fund.