Polyclonal gammopathy related to renal bleeding in a peritoneal dialysis patient
Article information
Abstract
Polyclonal gammopathy represents the diffuse activation of B cells and is usually related to inflammation or immune-related diseases. However, the mechanisms leading to polyclonal gammopathy are essentially speculative. Generally, infectious, inflammatory, or various other reactive processes may be indicated by the presence of a broad-based peak or band in the gamma region on serum protein electrophoresis results. A 15-year-old girl, who had been receiving peritoneal dialysis, presented with polyclonal gammopathy and massive gross hematuria. Renal artery embolization was performed, after which the continuous bleeding subsided and albumin-globulin dissociation resolved. This is a rare case of polyclonal gammopathy related to renal bleeding.
Introduction
Hypergammaglobulinemia results from the overproduction of immunoglobulins by several plasma cell lines1). Monoclonal gammopathy represents single plasma cell activation and is usually associated with several malignant diseases, including multiple myeloma, primary systemic amyloidosis, and other lymphoproliferative disorders1). In contrast, polyclonal gammopathy represents the diffuse activation of B cells, and is associated with a heterogeneous group of nonmalignant conditions, including inflammation and immune-related diseases1). We report a case of polyclonal gammopathy associated with massive gross hematuria in a pediatric patient who had bilateral dysplastic kidneys, and was undergoing peritoneal dialysis.
Case report
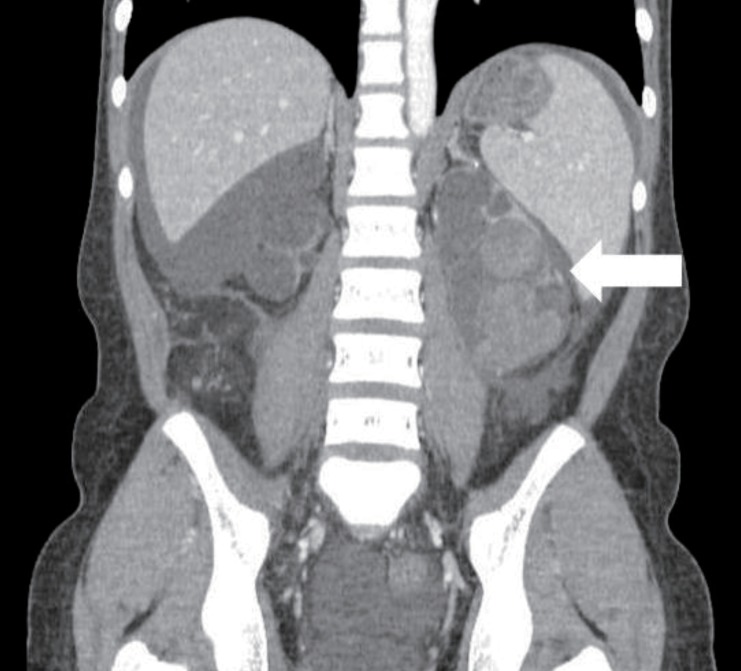
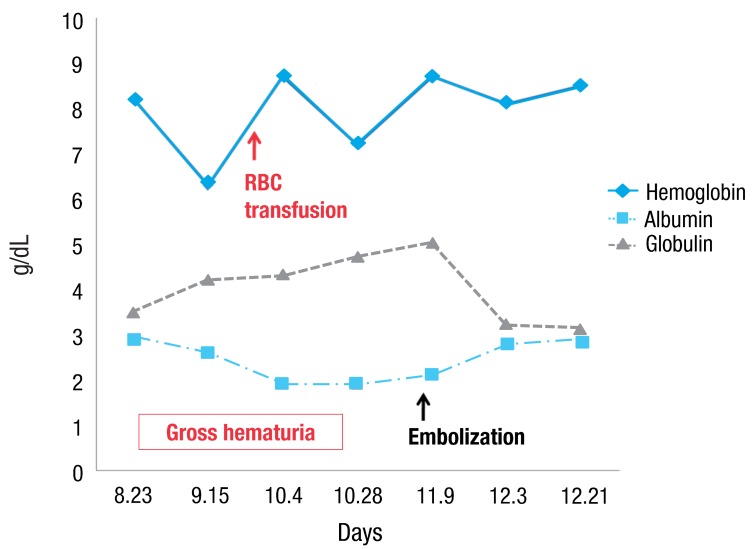
A-15-year-old girl was admitted with left flank pain and gross hematuria for several days. Four years ago, she was diagnosed as end-stage renal disease due to dysplastic kidneys which was multicystic dysplastic kidney in right side and was dysplastic kidney with severe hydronephrosis in left side. She underwent cadaveric kidney transplantation. However, because chronic allograft dysfunction has occurred, she has been undergoing peritoneal dialysis for 3 months. She had received oral immunosuppressant therapy during kidney transplantation state, and it was stopped when the peritoneal dialysis was started. Her blood pressure was 147/104 mmHg, heart rate was 112 beats/min and respiratory rate was 22 beats/min. She looked acutely ill and physical examination revealed severe tenderness on the left costovertebral angle, but no pitting edema. The laboratory findings on admission were hemoglobin 6.3 g/dL, hematocrit 18.7%, white blood cell count 5,640/µL (lymphocyte 78% neutrophil 17%) and platelet 144,000/µL. The blood chemistry showed total protein/albumin 6.8/2.6 g/dL, sodium/potassium 132/4.0 mmol/L, calcium/phosphorus 7.3/3.5 mg/dL, blood urea nitrogen/creatinine 43/13.1 mg/dL, erythrocyte sedimentation rate 53 mm/hr, and C-reactive protein 2.2 mg/dL. Coagulation blood test was prothrombin time 105%, partial thromboplastin time 30.5'', international normalized ratio 1.0. Her urine showed gross hematuria with blood clots and protein (4+) occult blood (3+) on dipstick. The analysis of dialysate was normal. Computerized tomography of her abdomen revealed encapsulated intrapelvic hematoma in the left kidney (Fig. 1). She was given red blood cell transfusion and with conservative therapy, flank pain and gross hematuria were improved. Follow-up serial hemoglobin level was remained and her blood pressure was maintained within the normal range. Follow-up abdominal computerized tomography showed as renal pelvic hematoma of the same size. She was discharged with improved condition. At the outpatient department, she had neither fever nor pain, but complained of intermittent gross hematuria with blood clot and her anemia was not improved. Ten days after discharge, follow-up laboratory findings showed a reversed albumino-globulin ratio (total protein, 6.6 g/dL; albumin, 1.9 g/dL; globulin, 4.7 g/dL; albumin/globulin ratio, 0.4) and this finding was sustained for two months in spite of no evidence of infection or inflammation. Serum electrophoresis was compatible with polyclonal gammopathy, which showed an increased component with alpha-1 and gamma fraction (Fig. 2). Thereafter, a massive gross hematuria was recurred and anemia was persisted. We performed the embolization of the left renal artery, two months after when the renal cystic hemorrhage began. The continuous bleeding was solved and also, albumino-globulin dissociation was normalized a month after renal artery embolization was done (Fig. 3).
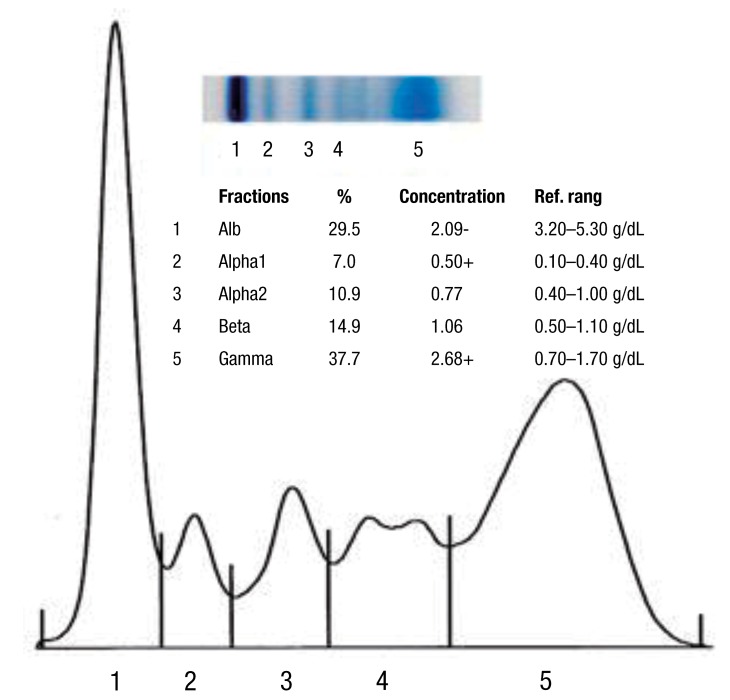
The result of serum protein electrophoresis showing elevated levels of alpha-1 and gamma globulin indicating polyclonal gammopathy. Alb, albumin; Alpha1, alpha-1 globulins; Alpha2, alpha-2 globulins; Beta, beta globulins; Gamma, gamma globulins.
Discussion
In general, patients with renal diseases, such as nephrotic syndrome, represent hypoalbuminemia and hypogammaglobulinemia. Serum globulin can be calculated by the total protein level minus the albumin level, and tends to increase in serious infectious diseases, inflammation, and malignancy. However, because globulin is composed of various protein components, simple change of globulin does not have meaningful diagnostic value. Albumin/globulin ratio (AG ratio) is one of the methods to easily detect the relative change of albumin and globulin and has a normal range between 1.5 and 2.2. For example, if there is an increase of albumin or decrease of globulin, AG ratio would be elevated above 2.2. On the contrary, AG ratio would be down less than 1.5, as in nephrotic syndrome. Because our patient showed increased globulin compatible with polyclonal gammopathy, as well as hypoalbuminemia, there was some discrepancy between our patient and patients with nephrotic syndrome. Serum electrophoresis can elucidate this situation.
Serum electrophoresis is a method for separating proteins, based on their physical properties. The net charge (positive or negative) and the size and shape of the protein commonly are used in differentiating various serum proteins2). Albumin is the major protein component of the serum and is produced by the liver, under normal physiologic condition. Globulins comprise a much smaller fraction of the total serum protein content. The alpha-1 fraction is comprised of alpha1-antitrypsin, thyroid-binding globulin, and transcortin, and it can be increased with specific condition, such as malignancy or inflammatio3). The alpha-2 component is increased as an acute phase reactants, beta-1 is composed mostly of transferring and beta-2 as beta-lipoprotein, is composed immunoglobulin (Ig) A, IgM, and sometimes IgG, along with complement proteins3). But much of the clinical interest is focused on the gamma region of the serum protein spectrum because immunoglobulins migrate to this region and can be found throughout the electrophoretic spectrum3). C-reactive protein is located in the area between the beta and gamma components2). Serum electrophoresis, in our patient, presented increased alpha-1 and predominantly, gamma fraction and it is consistent with acute or subacute inflammation. Gammopathy is an abnormal proliferation of the B lymphocytes, resulting in abnormal levels of immunoglobulin production. It is important to differentiate between a monoclonal and a polyclonal increase in immunoglobulins because the former is associated with a clonal process that is malignant or potentially malignant, while a polyclonal increase is due to a reactive or inflammatory process. Monoclonal gammopathy is an increased production of one type of immunoglobulin by a single clone of cells4). The abnormal protein produced is called paraprotein or M component and may be composed of whole immunoglobulin molecules or subunits, light-chains or heavy-chains4). It occurs usually in myelomas, lymphoproliferative neoplasms, and occasionally, chronic inflammatory or immune-mediated diseases5). Greatly elevated serum levels of protein may result in a hyperviscosity syndrome and it is more common in monoclonal gammopathy than the other one6). In contrast, polyclonal gammopathy is known to be caused by any reactive or inflammatory process, and is associated with nonmalignant conditions. Polyclonal gammopathy is a hypergammaglobulinemia, which results from an increased production of several different immunoglobulins. Infectious, inflammatory or various reactive processes may be associated with a broad-based peak or band in the gamma region in serum protein electrophoresis. Liver disease, autoimmune disease and chronic viral or bacterial infections may cause a polyclonal rise in the gamma fraction1,3). Pollock et al.7) surveyed 110 cases of renal transplant recipients and one half had abnormal serum immunoglobulins with 4 monoclonal gammopathy and 8 polyclonal gammopathy. Gammopathy was more common in patients with chronic rejection, and therefore, with more intense immunosuppression8). The mechanism leading to a post-transplant gammopathy in most cases appears to be related to diminution of T cell mediated immune surveillance, which leads to B cell proliferation9).
Then, what was the main cause of polyclonal gammopathy in our case? First, we assumed that immunosuppression itself could be the main cause. However, our patient didn't show this serologic problem during an aggressive immunosuppressive therapy after renal transplantation, and all immunosuppressive medicines were discontinued at the diagnosis on polyclonal gammopathy. Second, it was the possibility of polyclonal gammopathy, developed by a subclinical infection due to hematoma located in renal pelvis that can be a potential culture media. However, we could not discover the evidence of infection from any inflammatory markers and cultures. Another possible etiology is sustained hemorrhage from renal cysts. Actually, our patient used to show the erythropoietin resistant anemia, at the rebeginning of dialysis due to graft failure, and we did not find the evident cause of anemia. Retrospectively, it was possible that intrarenal bleeding was started before gross hematuria. When gross hematuria and pelvic hematoma were diagnosed initially, we considered that renal hemorrhage was not active and decided to delay the embolization of the renal artery. Although we cannot explain the precise mechanism of polyclonal gammopathy originated from renal hemorrhage, it supports that polyclonal gammopathy has disappeared when renal bleeding was controlled by embolization of the renal artery. We have experienced and taken notice of a case of polyclonal gammopathy in a peritoneal dialysis patient with renal cystic hemorrhage.
Notes
No potential conflict of interest relevant to this article was reported.