Implantable cardioverter defibrillator therapy in pediatric and congenital heart disease patients: a single tertiary center experience in Korea
Article information
Abstract
Purpose
The use of implantable cardioverter defibrillators (ICDs) to prevent sudden cardiac death is increasing in children and adolescents. This study investigated the use of ICDs in children with congenital heart disease.
Methods
This retrospective study was conducted on the clinical characteristics and effectiveness of ICD implantation at the department of pediatrics of a single tertiary center between 2007 and 2011.
Results
Fifteen patients underwent ICD implantation. Their mean age at the time of implantation was 14.5±5.4 years (range, 2 to 22 years). The follow-up duration was 28.9±20.4 months. The cause of ICD implantation was cardiac arrest in 7, sustained ventricular tachycardia in 6, and syncope in 2 patients. The underlying disorders were as follows: ionic channelopathy in 6 patients (long QT type 3 in 4, catecholaminergic polymorphic ventricular tachycardia [CPVT] in 1, and J wave syndrome in 1), cardiomyopathy in 5 patients, and postoperative congenital heart disease in 4 patients. ICD coils were implanted in the pericardial space in 2 children (ages 2 and 6 years). Five patients received appropriate ICD shock therapy, and 2 patients received inappropriate shocks due to supraventricular tachycardia. During follow-up, 2 patients required lead dysfunction-related revision. One patient with CPVT suffered from an ICD storm that was resolved using sympathetic denervation surgery.
Conclusion
The overall ICD outcome was acceptable in most pediatric patients. Early diagnosis and timely ICD implantation are recommended for preventing sudden death in high-risk children and patients with congenital heart disease.
Introduction
The annual incidence of sudden cardiac death in children is low, 1/100,000 individuals according to population based studies1). Arrhythmic causes are associated with electrical diseases such as ventricular tachycardia, ionic channelopathy2). Postoperative arrhythmia after repair of congenital heart disease is also one of the causes of sudden cardiac death in children3). To prevent sudden cardiac death, implantable cardioverter defibrillator (ICD) therapy has become an established standard care in adults. After the first ICD therapy in a young patient in 19894), it has been used with increasing frequency in children and in patients with congenital heart disease5). However pediatric ICD therapy has many limitations and only 1% of all ICD implantations are for pediatric patients6-8). Furthermore the experience of pediatric ICD implantation is very limited and there is only one published case report on pediatric ICD in Korea9). The purpose of this study was to document the clinical conditions requiring ICD implantation and effectiveness and limitations of ICD in children and congenital heart disease.
Materials and methods
We retrospectively reviewed the records of pediatric patients who underwent ICD implantation at Seoul National University Children's Hospital between 2007 and 2011. A total fifteen patients underwent ICD implantation during this period. Patient characteristics, indication of ICD implantation, implantation procedure, the effectiveness and complications were analyzed. Implant electrical parameter, type of lead and generator, defibrillation threshold, and initial R wave were also examined.
Two methods for ICD implantation were used, which were through the transvenous and epicardial routes. In the small children for the epicardial ICD implantation, we operated through the sternotomy and implanted a generator in the abdominal area.
The indication for the primary prevention of sudden death was the ICD implantation for cases that were at risk for but did not yet have an episode of sustained ventricular tachycardia (VT), ventricular fibrillation (VF), or resuscitated cardiac arrest. Secondary prevention of sudden death was for cases that had survived a prior sudden cardiac arrest or sustained VT.
All parameters were expressed as the mean±standard deviation.
Results
1. Patient's characteristics
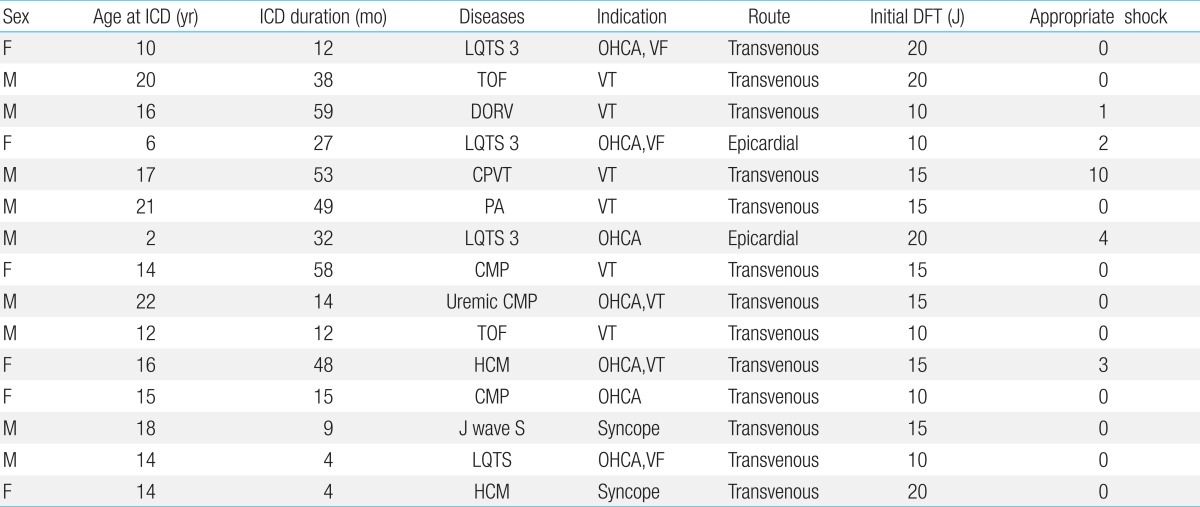
A total of 15 patients underwent ICD implantations. There were six females and nine males. The mean age at implantation was 14.5±5.4 years (range, 2 to 22 years). Additionally, the mean weight at implantation was 46.7±16.2 kg (range, 9.9 to 70 kg). The mean follow-up duration was 28.9±20.4 months (range, 4 to 59 months).
The underlying disorders were as follows: ionic channelopathy in 6 patients (long QT syndrome [LQTS] in 4 patients, catecholaminergic polymorphic ventricular tachycardia [CPVT] in 1 patient, and J wave syndrome in 1 patient); cardiomyopathy in 5 patient (hypertrophic cardiomyopathy in 2 patients, glycogen storage disease related cardiomyopathy in 1 patient, uremic cardiomyopathy in 1 patient, unspecified cardiomyopathy in 1 patient), and congenital heart disease in 4 patients (tetralogy of Fallot in 2 patients, double outlet right ventricle in 1 patient, pulmonary atresia with ventricular septal defect in 1 patient). Among the 4 LQTS patients, 3 patients were diagnosed by genetic testing, showing a type 3 LQTS with a SCN5A mutation. The most common underlying diagnosis was primarily electrical disease of ionic channelopathy.
2. Indication of ICD
The indication for ICD was cardiac arrest in 7 patients, sustained VT in 6 patients, and syncope in 2 patients (Table 1). Among the 15 patients, ICD was implanted as secondary prevention in 13 patients and as primary prevention in 2 patients. A patient with J wave syndrome, who received the ICD implantation as a primary prevention, had a strong family history of sudden cardiac death in young adults and he also had a history of syncope. During the electrophysiologic study, polymorphic VT was induced. The other patient with an ICD for primary prevention had hypertrophic cardiomyopathy with syncope and very severe septal hypertrophy.
Besides ventricular arrhythmias, associated arrhythmias were observed in 3 patients, sinus bradycardia in 2 patients, and atrial tachycardia in 2 patients.
3. Implantation procedures and types of ICD
The transvenous approach and the subpectoral positioning of active can were used in patients with a body weight more than 30 kg. In the younger children and those with less than 30 kg of body weight, the ICD was implanted via the epicardial route (Fig. 1).

Chest X-ray scan demonstrating the implantable cardioverter defibrillator (ICD) lead and device positions: (A) transvenous positioning of a dual-coil ICD lead in an adolescent; (B) epicardial positioning of the ICD lead within the pericardial sinus of a 6-year-old girl.
Among the 15 patients, 13 received the ICD through the transvenous route. The average age of the transvenous ICD group was 16.1 years old (range, 10 to 22 years) and their average weight was 52.7 kg (range, 30 to 70 kg). Instead of transvenous ICD, epicardial ICD implantation was used only for 2 patients. Their ages and weights were 2 and 6 years, and 9.9 and 26 kg respectively. For the epicardial ICD implantation, a single shock coil was placed at the pericardial transverse sinus using the transvenous ICD lead system after median sternotomy. A subrectus pocket was made in the upper abdominal wall to accommodate the ICD device (Fig. 1B).
Most patients had single chamber leads; however, 3 patients required double chamber leads with both atrial and ventricular leads due to associated sinus bradycardia in 2 LQTS patients and atrial tachycardia in a CPVT patient. Additionally, 7 patients received a double shock coil with a superior vena cava coil for down-regulating the defibrillation threshold. The mean initial defibrillation threshold (DFT) was 14.6±3.9 J (range, 10 to 20 J). The initial R wave was 9.8±3.9 mV and the latest checked R wave was 8.8±4.1 mV. In the case of the epicardial lead, the mean DFT was 10 and 20 J, and the R wave was 8.7 and 15.8 mV.
4. Effectiveness of ICD and follow-up
Five patients received the appropriate shock therapy for VT/VF for a total of 20 times (Table 1). In addition, the time interval between the ICD implantation to the first appropriate shock was 10.8±7.6 months (range, 3 to 24 months). Two patients with primary prevention have not received shock therapy for 12 months and 15 months of follow-up. On the other hand, a repaired tetralogy of Fallot patient and a double outlet right ventricle patient received inappropriate shocks due to sinus tachycardia and atrial tachycardia respectively. A beta blocker was effective in preventing further inappropriate ICD shocks in these two patients.
Some complications occurred in 4 patients (27%) among our study population. There were no procedure related complications. However two patients required revision of ICD lead for the reconnection or repositioning of leads. A patient with CPVT suffered from ICD storm because of persistent polymorphic VT. For the recurrent nonsustained VT in this patient, the VT detection time was lengthened and the thoracoscopic left sympathetic denervation surgery was added to minimize the ICD shock burden.
Despite ICD implantation, concomitant medication was needed. Beta blockers such as atenolol, carvedilol, and propranolol were used in 13 patients and sotalol in 2 patients. A patient with J wave syndrome received no medication. Among our patients, one died because of intractable heart failure. He was the youngest ICD case and genetically diagnosed as LQTS type 3 with dilated cardiomyopathy9). There was no arrhythmia related sudden death during a follow-up period of 28.9 months (range, 4 to 59 months).
Discussion
This study describes the early experience of pediatric ICD in Korea. Since 1996, the first case report of adult ICD implantation in Korea10), ICD has been applied increasingly in high risk adult population to prevent sudden cardiac death. However the experience of ICD therapy in children and congenital heart disease patients is limited and there is only one pediatric case report9), which case has been enrolled in this study. We have applied ICD for primary prevention in 2 patients and secondary prevention in 13 patients. And the short term follow-up result was acceptable. Among 15 applied patients, 5 patients received the appropriate ICD shock therapy for 28.9±20.4 months (range, 4 to 59 months) of follow-up. In two young children (2 and 6 years old), ICD coils were implanted in the pericardial space.
In approximately two thirds of sudden death in children and young adult, the underlying cardiac disease can be identified1). The frequently identified cardiac causes are hypertrophic cardiomyopathy, myocarditis, coronary artery abnormality, conduction system anomalies, and congenital heart disease2,3). In an international multi-institutional study on LQTS, the overall incidence of sudden death was 8% and for hypertrophic cardiomyopathy, the risk of sudden cardiac death was about 4%11,12).
In contrast to ICD indications in adult ICD populations, children and young adults with ICD can be classified among three main categories of heart disease: ionic channelopathy, cardiomyopathy, and congenital heart disease. During the past 15 years, the frequency of ICD implantation has been changed. In one of the earliest studies in 1993, a majority of the 125 pediatric patients had cardiomyopathy (54%), followed by ionic channelopathy (26%), and congenital heart disease (18%)13). The recent publications show there is a tendency of increased implantations in those with ionic channelopathy and congenital heart disease5,14). The underlying disorders of this study were similar to those of western countries.
In the past, ICD implantation in children and young adults has been reserved for secondary prevention. However, more recently the primary prevention has increased from 15% to almost 50%5,13-16). This may reflect the impact of genetic testing on the decision making regarding device implantation, improved device technology, and the increased recognition of the risk of sudden death. The proportion of primary prevention in this study was lower than those of western countries. The reasons of this difference may be the difference of genetic background, late introduction of surgical correction of congenital heart disease, and the medico-social tendency of delaying ICD therapy in children in our country. As an ICD lead system, transvenous insertion is suitable for older children and adults. However, this system is not suitable in younger children and infants due to their small size and increased potential in venous obstruction. Furthermore, in children with congenital heart disease such as single-ventricle defects palliated with Fontan connections, cardiac and venous anatomy may prohibit a transvenous approach. Thus, an epicardial patch and subcutaneous ICD system are the two common alternatives used in children when a transvenous approach is undesirable or not feasible13-16). Epicardial patch ICD systems require a full sternotomy or thoracotomy and can lead to constrictive pericarditis. In addition, crinkling and failure of the epicardial patch can result in an increase in the DFT that requires lead revision in up to 25% of patients. A subcutaneous placement of an ICD lead or array in the chest wall avoids extensive operative exposure but may require at least a partial sternotomy for epicardial sense-pace electrode placement. And in comparison to the transvenous approach, the epicardial patch or subcutaneous lead system has shown a problem of increase in the DFT16-19). In 2 young children of 2 and 6 years old in this study, the ICD shock coils were implanted at the transverse sinus pericardially after median sternotomy. For epicardial approach, we used ICD shock coil which had been originally developed for transvenous application. Although the follow-up duration was short, the DFT was acceptable and there were several appropriate shock therapies in these 2 children. Five patients received the appropriate ICD shock therapies and two patients received inappropriate shocks due to supraventricular tachycardia. All appropriate therapies were observed in the patients for secondary prevention. Inappropriate therapy is usually triggered by sinus tachycardia, supraventricular tachycardia, T wave over-sensing and lead complications20,21). An inappropriate ICD discharge results in significant morbidity, occurring in 13% to 40%, of young ICD patients within 4 years of implantation. This frequent occurrence might be attributed to higher heart rates, earlier lead failures related to increased activity, somatic growth, lead stress, and a longer life span with the ICD22).
The limitations of this study include its retrospective nature, the relatively short-term data collection, and the small sample size. However, since this is the first analysis of the current pediatric ICD experience in Korea, our results may be noteworthy.
In summary, ICD was a safe and reliable therapy for resuscitated sudden death and syncope due to VT to prevent sudden death. The intrapericardial ICD lead positioning can be considered in small children who need epicardial ICD implantation. Early diagnosis and timely ICD implantation are recommended to prevent sudden death in high risk children and congenital heart disease patients.
Notes
No potential conflict of interest relevant to this article was reported.