Selenium and leptin levels in febrile seizure: a case-control study in children
Article information
Abstract
Purpose
Febrile seizures (FS) are seizures that occur between the age of 6 and 60 months, but its pathophysiology still is not fully understood. There is limited information about the correlation between levels of selenium and leptin with FS. This study aimed to determine the relationship between serum levels of selenium and leptin in children with FS.
Methods
This case-control study was conducted in a University Hospital in Shahrekord, Iran, in 2011. The serum levels of selenium and leptin of 25 children with simple febrile seizure (case group) were compared with 25 febrile children without seizure (control group) in acute phase and after three months. The levels of serum selenium and leptin were measured by flame atomic absorption spectrophotometer and enzyme-linked immunosorbent assay method, respectively.
Results
In acute phase, the mean serum level of selenium in case and control groups were 95.88±42.55 and 113.25±54.43 µg/dL, respectively, and difference was not significant (P=0.415), but after three months, this level had a significant increase in both groups (P<0.001). In acute phase, the mean serum leptin level in case and control groups were 0.94±0.5 and 0.98±0.84 ng/mL, respectively, but difference was not significant (P=0.405). After three months, serum leptin level had no significant change in both groups (P=0.882).
Conclusion
These observations suggest that serum levels of selenium and leptin have not specific relation with FS but overllay is lower, however, further study is recommended. Also selenium level in stress and acute phase was significantly lower than recovery phase.
Introduction
Febrile seizures (FS) are seizures that occur between the age of 6 and 60 months with a temperature of 38℃ or higher, that are not the result of central nervous system infection or any metabolic imbalance, and that occur in the absence of a history of prior afebrile seizures1). The incidence of FS is between 2% to 5%2). Although febrile seizure is the most common form of seizures in children3), its pathophysiology still is not fully understood4). Risk factors for FS include genetic factors, micronutrient deficiency e.g., iron, zinc3,5,6), and immunologic reactions7).
It is about one century since the addition of selenium to the periodic table of elements. After 1973 when Rotruck et al.8) revealed the role of selenium in a detoxifying enzyme, glutathione peroxidase, this element has been demonstrated to have a positive biological function in various aspects of human health. Selenium is an important micronutrient that has antioxidant effects in cells, especially in brain cells9,10). Also studies have shown that seizures may result in free radical production and oxidative damage11).
Mahyar et al.12) and Amiri et al.13) showed that the serum selenium level in the children who had simple FS was significantly lower than in the non-seizure control group.
Leptin, a 16 kDa neurohormone predominantly synthesized and released into blood by adipocytes, has an important role in regulating body weight and has receptors on neurons outside the hypothalamus and modulates their excitability acutely. Leptin modulation of neuronal excitability outside the hypothalamus is potentially important if blood leptin can access its receptors on these neurons. Leptin transporters throughout the brain give leptin access to its receptors on hippocampal and other cortical neurons14).
The increased serum leptin in rats fed a ketogenic diet further implicates leptin as an endogenous modulator of neuronal excitability because the ketogenic diet is an effective treatment for intractable epilepsy15,16). This observation suggests that chronically elevated blood leptin levels decrease neuronal excitability17).
In contrast to the anticonvulsant effects that have been reported for leptin in some nonclinical studies, a number of investigators have described opposite findings: i.e., proconvulsant and convulsant-related activity. For example, leptin application was found to be neuroexcitatory in that it enhanced N-methyl-D-aspartic acid (NMDA) receptor-mediated increases in intracellular Ca2+ levels and synaptic transmission in rat hippocampal cell cultures and brain slices18). Using electrophysiological recordings from proopiomelanocortin type neurons of mice, it was demonstrated that leptin increased the frequency of action potentials19). In dissociated cerebellar granule cells, leptin augmented Ca2+ influx following activation of NMDA receptors20). Additionally, in vivo administration of leptin to rats enhanced the rate of penicillin induced epileptiform activity, thus leading the investigators to speculate that leptin could have proconvulsant activity21). So a number of other nonhuman experiments have demonstrated proconvulsant activity for leptin22). A possible role of selenium and leptin in provoking FS has been reported in a few studies12,13), but it is still questionable whether selenium and leptin play any role in the provoking of FS. The present study was designed to determine the correlation between serum levels of selenium and leptin and FS.
Materials and methods
This case-control study was conducted at the hospital of the University of Shahrekord, Iran. The study was approved by the Ethical Committee of the Research Department in the Shahrekord University of Medical Sciences before data were collected.
For this study, we enrolled 25 children who were clinically diagnosed with FS. All FS patients met stringent FS criteria based on those used by Freeman23). Twenty-five febrile children without seizure for controls were recruited from febrile patients who were admitted to pediatric department for non-FS diseases such as pneumonia or acute gastroenteritis. The case and control were selected consecutively from children admitted to the hospital, based on their symptoms. Inclusion criteria for the case group were age between 6 to 60 months; simple febrile seizure; absence of central nervous system infection; and absence of electrolyte imbalance. Children with complex FS, no febrile seizure, epilepsy, and neurologic deficit were excluded.
For each patient, the following information was obtained: complete medical history and clinical examination with special emphasis on age, sex, type and frequency of seizure and duration of illness. Inclusion criteria for the control group were fever without a history of seizure, admitted to hospital because of mild infection, and no intervention for their problem. Children in both groups were matched in terms of age, sex, weight, height, head circumference, and fever severity.
Weight, height, head circumference, and body temperature (axillary) were measured according to standard methods. None of the individuals showed any digestive symptoms indicative of nutrient malabsorption. All parents of the children were given information about the research method in a simple language and had signed the written informed consent form.
The serum levels of selenium and leptin of twenty-five children with simple FS (case group) were compared with twenty-five febrile children without any history of FS (control group) in acute phase (at least 12 hours after admission) and recovery phase (after three months). The study was conducted from 2010 to 2011.
Blood samples were taken between 9 AM and 11 AM. In the case group, the blood sample was taken at least 12 hours after treating the seizure. In the both groups, 6 mL blood from the peripheral vessels was taken and centrifuged. The clean serum was then poured into an acid-washed tube and stored at -70℃ in the refrigerator until the time of analysis. Selenium concentration was determined using hydride generation atomic absorption spectrometry. Measurement of serum selenium was done by an atomic flame spectrophotometer method. The flame spectrophotometer machine was an Australian-made Varian Spectra AA220 model. To improve accuracy, all measurements of serum selenium were rechecked. The normal range of serum selenium level is 46.143 µg/dL24). Leptin was detected with commercially available test kits were used to measure leptin (enzyme-linked immunosorbent assay [ELISA] method, DRG International, Mountainside, NJ, USA), which has a range of 0.25.120 ng/mL. The DRG Leptin ELISA is an enzyme immunoassay for the measurement of leptin in serum and plasma. In the DRG Leptin ELISA the following values are observed: males 3.84±1.79 ng/mL, females 7.36±3.73 ng/mL25).
1. Statistical analysis
Data are shown as mean±standard deviation. All statistical analyses were performed using the IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). In addition, Mann-Whitney, Wilcoxon, and chi-square tests were used to compare the variables between the both case and control groups. A value of P<0.05 was considered significant.
Results
This study enrolled twenty-five Iranian patients who had been clinically diagnosed with FS and twenty-five febrile children without any history of FS. The average ages of the FS and control patients, were 24.8±13.7 months, and 24.5±13.9 months, respectively and there was no statistically significant difference between the groups (P=0.8). In the case group, 18 (72%) were male and 7 (28%) were female; in the control group, 21 (84%) were male and 4 (16%) were female and there was no statistically significant difference between the groups (P=0.3).
The average body mass index of the FS and control patients, were 17.46±2.63 and 16.37±1.97, respectively and there was no statistically significant difference between the groups (P=0.1).
In acute phase, minimum and maximum level of serum selenium in the case group was 18.4 and 186.2 µg/dL, respectively, compared with 47.44 and 240.54 µg/dL in the control group. In this time, minimum and maximum level of serum leptin in the case group was 0.4 and 2.2 ng/mL, respectively, compared with 0.3 and 3.5 ng/mL in the control group.
After three months, minimum and maximum level of serum selenium in the case group was 99.14 and 277.54 µg/dL, respectively, compared with 68.24 and 496.24 µg/dL in the control group. In this time, minimum and maximum level of serum leptin in the case group was 0.4 and 2.1 ng/mL, respectively, compared with 0.3 and 1.7 ng/mL in the control group.
The mean selenium and leptin levels in case group were 95.88±4.25 µg/dL and 0.94±0.5 ng/mL respectively, and in control group, were 113.25±5.44 µg/dL and 0.98±0.84 ng/mL, respectively. Mann-Whitney test showed no significant differences in the levels of selenium and leptin between patients in case and control groups, although patients with FS had lower levels of selenium and leptin than the control group (P>0.05). After three months, Wilcoxon U test showed selenium level had a significant increase in both groups (P<0.001), but leptin level had not significant change in both groups (P=0.882) (Table 1).
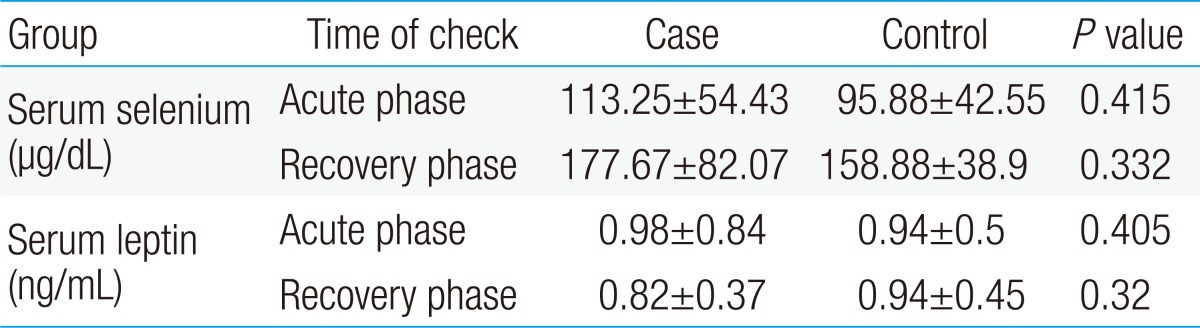
Serum selenium and leptin levels in the case and control groups in the acute and recovery phases (After 3 Months)
Twenty-one of the twenty five FS patients (84%) had only one FS attacks by the time of our study.
Discussion
Febrile seizure is the most common brain-related disease in children3). Several studies have indicated that multiple factors can be involved in the pathogenesis of FS. This study was designed to address whether selenium and leptin have any role in developing FS.
Oxidative stress and production of reactive oxygen and nitrogen species are some of the etiologies of epilepsy26-28). Selenium was used in management a number of the neurologic disorders including epilepsy in some studies29,30). Selenium has antioxidant properties; it prevents cell bodies from oxidation and keeps them biologically healthy31).
Although the correlation between selenium deficiency and epilepsy has been shown in several studies32,33), the pathogenesis involvement of selenium in FS has not been completely recognized.
Simple FS are generally considered as benign phenomenon, with tendency to develop epilepsy, especially the temporal form of seizure following a prolonged seizure34).
Although the pathophysiology of FS is still unknown1,13,16), several studies have indicated that multiple factors can be involved in the pathogenesis of FS, including genetic factors3) interleukin-1β35), iron36), and zinc37,38).
It is known that oxidative stress and generation of reactive oxygen species are a cause and consequence of epileptic seizures. It has been suggested that some antiepileptic drug therapies such as valproic acid, deplete the total body selenium level and selenium-dependent glutathione peroxidase (GSH-Px) activity although therapy with a new epileptic drug, topiramate, activated GSH-Px activity in epileptic animals and humans. Selenium is incorporated into an interesting class of molecules known as selenoproteins that contain the modified amino acid, selenocysteine. There are signs of selenium and selenoprotein deficiency in the pathogenesis of epilepsy39).
In summary, there is convincing evidence for the proposed crucial role of selenium and deficiency of GSH-Px enzyme activity in epilepsy pathogenesis40).
Mahyar et al.12) and Amiri et al.13) found that serum selenium level in the children with febrile seizure was significantly lower than that in healthy children, but in the recent study there was no significant difference in serum selenium level in the children with FS and non-FS children and there was no significant difference between genders. Serum selenium level in this study was similar to Safaralizadeh et al.40) research in Tehran, but higher than Mahyar et al.12) and Amiri et al.13). Difference between these studies probably related to geographical difference in intake of selenium in different regions. There is no integrated study in Iran about selenium level in different geographic areas, but results of present study revealed that selenium level in Shahrekord (South West Iran) may be higher than Qazvin and Tehran12,13).
In present study selenium level was assessed in two stages: acute and recovery phase, and in both stage, the average level was higher than Mahyar et al.12) and Amiri et al.13) study. Differences in selenium level related to several factors such as selenium content of soil, dietary habits and the geographical variation of selenium intake41). In some regions of the world such as Finland, New Zealand, the East Coast of the United States of America, and China the content of selenium in soil is remarkably low42). Therefore selenium levels in the serum of populations throughout the world are different. There is currently no information of selenium intake and serum levels in the Iranian population, so difference in serum selenium levels in Iranian study may be related to content of selenium in soil.
Similar to Sammalkorpi et al.43), Srinivas et al.44), and Olmez et al.45) findings, after three months, in recovery period, selenium level had a significant increase in both groups of study. Selenium, as an essential micronutrient, is required for the proper functioning of the immune system and its deficiency affects the occurrence, virulence, or disease progression of some viral infections. Sammalkorpi et al.43) concluded that acute infections decrease serum levels regardless of the infective agent, because of the possible connection between selenium and the immune system.
Measurement of micronutrient levels in the presence of inflammation is difficult for several reasons. Changes in levels of acute phase proteins are associated with increased plasma levels of some indicators of micronutrient status, such as ferritin, and decrease of others, such as retinol. Alterations in the plasma levels of acute phase proteins can occur from hemodilution, sequestration and increased or decreased rates of synthesis and breakdown. How much these relate to functional deficiency is not known46). In other hand, selenium level as a micronutrient, is often measured directly in serum, plasma, whole blood and hair. It has also been measured indirectly by assays of plasma GSH-Px levels47). Both serum and whole blood levels are decreased during the acute phase response. Low levels occur in several infections43,48). Several viral infections appear to stimulate the production of selenoproteins, leading to low serum levels of selenium. Patients with the lowest levels of selenium have the highest levels of morbidity and mortality but a causal effect has not yet been demonstrated49). Overall it is noted that selenium levels are low in infection. It is due to the consumption of selenium as an antioxidant as part of the process of quenching free radicals50). Whether plasma selenium is also reduced as part of the inflammatory process has not been studied intensively and there are no published attempts to control for changes in selenium due to changes in inflammatory proteins.
Additionally, the mean leptin value in case and control group at baseline of recent study was 0.94±0.5 and 0.98±0.84 ng/mL, respectively. Although patients with FS had not significant lower levels of leptin than the control group, after three months, leptin level had not significant change in two groups.
To our knowledge, however, there have been no similar studies establishing the correlation between serum leptin level and FS. Leptin has been proposed for clinical use as an anticonvulsant in addition to its potential metabolic effects in humans51-53). Support for such an indication has come from both in vitro and animal study data. For example, Glaum et al.54) demonstrated that leptin treatment reduced evoked glutamatergic excitatory postsynaptic current (an antiexcitatory effect) in rat brain slices but had no effects on GABAergic inhibitory postsynaptic current. In rat hippocampal brain cell cultures and slices, leptin exposure decreased evoked epileptiform-like activity52,55). Furthermore, an in vivo study demonstrated that leptin inhibited 4-aminopyridine-induced convulsions in rats and pentylenetetrazole-induced convulsions in mice, and another study showed that pentylenetetrazole-induced convulsions were less severe in wild-type mice than in leptin-deficient mice53,56).
Evaluation of leptin levels in response to experimental models of inflammation in rats revealed elevated plasma concentrations57). Leptin was also shown to be involved in the early (<24 hour) acute phase response after moderately severe surgical trauma58), and to play a role in acute sepsis59). Its significant correlation with other acute-phase proteins indicates that leptin could be a participant in acute-phase protein synthesis regulation during a systemic inflammatory response60).
In contrast to the anticonvulsant effects that have been reported for leptin in some nonclinical studies, a number of investigators have described opposite findings: i.e., proconvulsant and convulsant-related activity. For example, leptin application was found to be neuroexcitatory in that it enhanced NMDA receptor-mediated increases in intracellular Ca2+ levels and synaptic transmission in rat hippocampal cell cultures and brain slices18). Using electrophysiological recordings from proopiomelanocortin type neurons of mice, it was demonstrated that leptin increased the frequency of action potentials57). Additionally, in vivo administration of leptin to rats enhanced the rate of penicillin-induced epileptiform activity, thus leading the investigators to speculate that leptin could have proconvulsant activity26).
In summary, results of the present study indicate that, the serum selenium and leptin levels of children with simple FS were not significantly lower than that of febrile children without seizure and other factors may be involved. In addition, studies with larger samples are recommended to further examine the correlation between FS and the levels of serum selenium and leptin.
Limitations to the present study (low budget and low sample size) influence the generalizability of the results. Further research with larger samples and healthy control group is warranted to identify the correlation of selenium and leptin with FS.
Acknowledgments
The authors thank all the participants and others who helped in completing this study. This study was registered in the research department of the Shahrekord University of Medical Sciences. The authors received a grant from the research department of Shahrekord University of Medical Sciences to conduct this research.