Right ventricular failure in congenital heart disease
Article information
Abstract
Despite developments in surgical techniques and other interventions, right ventricular (RV) failure remains an important clinical problem in several congenital heart diseases (CHD). RV function is one of the most important predictors of mortality and morbidity in patients with CHD. RV failure is a progressive disorder that begins with myocardial injury or stress, neurohormonal activation, cytokine activation, altered gene expression, and ventricular remodeling. Pressure-overload RV failure caused by RV outflow tract obstruction after total correction of tetralogy of Fallot, pulmonary stenosis, atrial switch operation for transposition of the great arteries, congenitally corrected transposition of the great arteries, and systemic RV failure after the Fontan operation. Volume-overload RV failure may be caused by atrial septal defect, pulmonary regurgitation, or tricuspid regurgitation. Although the measurement of RV function is difficult because of many reasons, the right ventricle can be evaluated using both imaging and functional modalities. In clinical practice, echocardiography is the primary mode for the evaluation of RV structure and function. Cardiac magnetic resonance imaging is increasingly used for evaluating RV structure and function. A comprehensive evaluation of RV function may lead to early and optimal management of RV failure in patients with CHD.
Introduction
Progress in new surgical techniques and medical management for congenital heart diseases (CHD) has dramatically improved patient survival over the past decades. However, with many patients with CHD surviving until adulthood, right ventricular (RV) failure has become a concern1). Several types of CHD are associated with RV failure, although surgical or interventional adjustments have been developed for CHD1). RV outflow tract (RVOT) obstruction after total correction of the tetralogy of Fallot (TOF), pulmonary stenosis, atrial switch operation for transposition of the great arteries (TGA), congenitally corrected TGA (ccTGA), and systemic RV failure after the Fontan operation are the causes of pressure-overload RV failure1,2). Another problem is the volume-overload RV failure that may be caused by atrial septal defect (ASD), pulmonary regurgitation, and tricuspid regurgitation1,2). The development of RV failure associated with CHD should be carefully monitored, and both optimal medical and surgical treatments should be considered. The aim of this review is to provide an update on the current understanding of RV failure in patients with CHD.
RV anatomy
In contrast to the ellipsoidal shape of the left ventricle (LV), in the sideward view, the right ventricle appears triangular, and in the cross-sectional view, it appears crescent shaped3,4). The right ventricle can be divided into 3 components: 1) the inlet, 2) the trabeculated apical myocardium, and 3) the infundibulum or conus5) (Fig. 1). The specific morphological features of the anatomy of the right ventricle include the following: 1) the more apical attachment of the septal leaflet of the tricuspid valve relative to the anterior leaflet of the mitral valve, 2) the presence of a moderator band, 3) the presence of >3 papillary muscles, 4) the trileaflet of the tricuspid valve with septal papillary attachments, and 5) the presence of prominent and coarse trabeculations3).
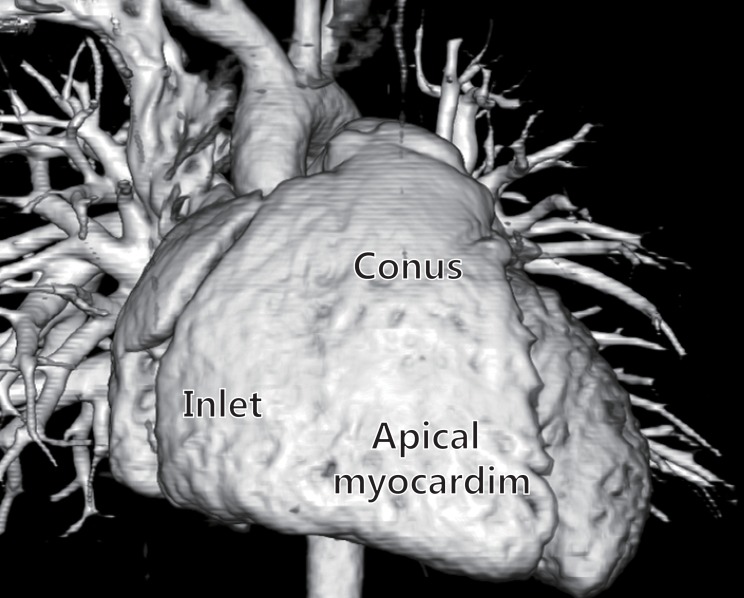
Three-dimensional computed tomography images of a normal heart showing the inlet, trabeculated apical myocardium, and infundibulum of the right ventricle.
Although the right ventricle appears smaller than the LV in the 4-chamber view, the volume of the right ventricle is more than that of the LV4,6). In normal adults, RV mass is only approximately one-sixth that of the LV, and the right ventricle has a wall thickness 3 to 4 times less than that of the LV7). Progressive regression of RV hypertrophy is observed as pulmonary vascular resistance (PVR) decreases during childhood4).
The right ventricle is linked to the LV at several points such as a shared ventricular septal wall, mutually encircling epicardial fibers, attachment of the RV free wall to the anterior and posterior septum, and sharing the pericardial space2).
RV physiology
The essential function of the right ventricle is to receive systemic venous blood and pump it into the pulmonary arteries. In the absence of shunt physiology or significant valvular regurgitation, the right ventricle pumps the same stroke volume as the LV4). However, the stroke work of the right ventricle is only approximately 25% of that of the LV because of low vascular resistance and pulmonary artery distensibility. Therefore, the right ventircle is thinner walled and more compliant2-4) than the LV. RV contraction starts with the inlet and trabeculated myocardium and ends with the infundibulum3). In contrast to the LV, twisting and rotational movements do not significantly contract the right ventricle, and RV shortening is greater longitudinally than radially3,8). RV systolic function is a reflection of contractility, afterload, and preload. RV performance is also influenced by heart rhythm, synchrony of ventricular contraction, RV force-interval relationship, and ventricular interdependence9-12). Compared with the LV, the right ventricle demonstrates a heightened sensitivity to afterload change3,13,14). In clinical practice, PVR is the most commonly used index of afterload3). The PVR is influenced by hypoxia or hypercarbia, cardiac output, pulmonary volume and pressure, and specific molecular pathways such as the nitric oxide, prostaglandin, and endothelin pathways3,15,16). Excessive RV volume can compress the LV and impair global LV function through the effects of ventricular interdependence14). The main structures for ventricular interdependence include the ventricular septum, pericardium, and continuity between myocardial fibers of the right ventricle and LV4). In acute RV pressure- or volume-overload states, dilatation of the right ventricle shifts the interventricular septum toward the left, altering LV geometry3). This leads to a decreased LV preload, an increased LV end-diastolic pressure, or low cardiac output12,15).
The pressure-overloaded right ventricle
When the right ventricle is exposed to pressure overload, progressive dilation follows a primary adaptive response that includes hypertrophy2). Although some reports state that hypertrophy itself is beneficial for overcoming the increased backward pressure, it is well accepted that a persistent increase in the pressure of the right ventricle causes a loss of contractile force that is required for pumping out the blood17,18). Moreover, uncorrected RV failure often induces diastolic dysfunction of the LV2). Pressure overload of the right ventricle also may lead to RV ischemia, which may further aggravate ventricular dysfunction14). Compared with the volume-overload condition, histological changes are more common in the RV pressure-overload condition, in particular, increased myocardial fibrosis, which is seen in both animal and human studies19,20). Two major conditions of pressure loading of the right ventricle are RVOT obstruction and the right ventricle supporting the systemic circulation.
Isolated pulmonary stenosis is the most common RVOT obstructive CHD. Although the obstruction may also occur at the subvalvar or supravalvar levels, 80% to 90% of cases have valve level obstruction1). Regardless of the level of obstruction, the right ventricle exerts a hypertrophic response according to the degree of obstruction21). The pressure gradient across the RVOT can be estimated by continuous wave Doppler echocardiography, which correlates well with catheter-based peak-to-peak gradient, obviating the need for cardiac catheterization1,22).
In patients with moderate-to-severe pulmonary valve stenosis, symptoms are uncommon before adulthood1). The right ventricle usually adapts well to pulmonary valve stenosis, even when the stenosis is severe. Longstanding untreated severe obstruction, however, may lead to RV failure and tricuspid regurgitation1,4). Percutaneous valvuloplasty is considered in patients with moderate-to-severe pulmonary valve stenosis4).
In terms of physiology and anatomy, the right ventricle has obvious disadvantages involved in supporting the systemic circulation. It is well suited to changes but is poorly tolerant to acute changes in afterload1,7). Late RV failure usually occurs in patients with TGA who have undergone an atrial switch surgery and in patients with ccTGA, because the anatomy of the right ventricle supports the systemic circulation1,23). The cause of RV dysfunction is unclear. However, myocardial perfusion defects, uncoordinated myocardial contraction, and systemic atrioventricular valve (tricuspid valve) regurgitation contribute to the progressive decline in RV function in patients who have undergone an atrial switch operation1,23,24). RV dilatation and impaired systolic function correlates inversely with RV systolic function25).
In patients with ccTGA, long-term outcome is abnormal even in patients without associated lesions. Moderate-to-severe systemic atrioventricular valve (tricuspid valve) regurgitation and RV failure are associated with increased mortality1,4,26). RV dysfunction usually starts within 5 years from the onset of TR in ccTGA patients without associated lesions2). RV failure with ventricular enlargement also results in TR aggravation due to annular dilatation. Tricuspid valve replacement may slow the progression of RV failure18). Among the older patients with ccTGA, many may be considered for mechanical support or heart transplantation3).
The volume-overloaded right ventricle
The right ventricle adapts better to volume overload than to pressure overload18) and may tolerate volume overload for a long time without significant systolic dysfunction1). Recent studies, however, have demonstrated that chronic volume overload is associated with increased morbidity and mortality1).
A large ASD results in left-to-right shunting and volume overload of the right ventricle18). Large ASDs may remain minimally symptomatic during the high-volume phase, until and Eisenmenger's syndrome and pulmonary vasculopathy develop2). In contrast to patients with ventricular septal defects, only a small percentage of patients with ASD develop Eisenmenger's syndrome in later life27). Age older than 40 years at closure is associated with incomplete right ventricle, right atrial reverse remodeling, and increased risk of arrhythmias1,27). Closure of the ASD is contraindicated in patients with Eisenmenger physiology, unless significant regression of pulmonary vascular disease occurs with pharmacological therapy3).
Severe pulmonary regurgitation is the most common cause of progressive RV dilatation and dysfunction in patients with repaired TOF1). It is associated with decreased exercise tolerance, arrhythmias, and sudden death1). Severe and progressive RV dilatation may be a primary sign of a RV dysfunction, an indication for pulmonary valve replacement. Pulmonary valve replacement generally results in ventricular reverse remodeling with a decrease in RV volume1). Advanced severe RV dilatation with an end-diastolic volume >170 mL/m2 or an end-systolic volume >85 mL/m2 before replacement, however, is associated with persistence of RV dilatation after surgery28). In TOF, a "restrictive RV physiology" has been associated with worse outcome after repair of TOF1). A restrictive RV physiology is characterized by the presence of forward and laminar late diastolic pulmonary flow throughout respiration1). Early after TOF repair, restrictive RV physiology is associated with a low cardiac output and longer intensive care unit stay1,29). Late after TOF repair, however, restrictive RV physiology counteracts the effects of chronic pulmonary regurgitation1,30).
Ebstein anomaly is characterized by an apical displacement of the septal and posterior tricuspid leaflets exceeding 8 mm/m21,31), leading to an atrialized RV and moderate-to-severe tricuspid regurgitation. RV failure in Ebstein anomaly results from volume overload of the right ventricle and from a hypoplastic RV chamber unable to manage the systemic venous blood18). In symptomatic patients, the size of the functional RV and tricuspid valve morphology determine the best surgical technique1).
Assessment of the right ventricle
Although the measurement of RV function is difficult, the right ventricle can be evaluated using several imaging and functional modalities2,18). In clinical practice, echocardiography is the mainstay for evaluating the RV structure and function18). Compared with other modalities, it is versatile and available at all institutes18). In addition, Doppler-derived indices such as the myocardial performance index (MPI) and tricuspid annular isovolumic acceleration (IVA) are emerging as promising parameters of RV function18). Cardiac magnetic resonance imaging (MRI) is the primary technique for evaluating RV structure and function18). MRI is considered the most accurate tool for assessing RV volume18). MRI may have an extending future role in assessing the physiological characteristics of pulmonary arterial flow18). Radionuclide-based techniques provide reliable and geometrically independent assessments of RV ejection fraction (RVEF)18). Radionuclide based time activity curves are also useful in the quantification of shunts16). Cardiac catheterization provides direct hemodynamic data and allows accurate assessment of PVR. Pulmonary angiography and coronary angiography can further delineate important anatomic and functional characteristics18). The simplest method for assessing RV volume includes linear dimensions and areas obtained from single tomographic echocardiographic planes18). In an effort to be more accurate, different approaches have been sought to measure RV volume. These include the area-length method and the Simpson's rule method18). Three-dimensional echocardiography is a promising technique that could lead to more accurate measurement of RV volume18).
The study of RV function comprises indices that reflect RV systolic function, RV diastolic function, and valvular function2,32). The most commonly used echocardiographic indices of RV systolic function are as follows: 1) geometric indices such as RV fractional area change, RVEF, and tricuspid annular plane systolic excursion (Fig. 2), which reflect the extent of contraction; 2) myocardial velocity indices such as the tricuspid annular plane maximal systolic velocity and the IVA (Fig. 3); 3) hemodynamic indices such as the first RV derivative of pressure and time (RV dP/dt); and 4) time interval indices such as the RV MPI (Fig. 4) or Tei index, which reflect both systolic and diastolic parameters33). Right atrial pressure (RAP) is a clinically useful diastolic variable of RV diastolic function34). In patients who are not being mechanically ventilated, the inferior vena cava size and collapse index correlate well with RAP4). Recent studies showed that serum levels of B-type natriuretic peptide may be useful in diagnosing RV failure associated with CHD35,36). Elevated troponin levels have also been associated with worse outcomes in pulmonary embolism and pulmonary hypertension37,38).
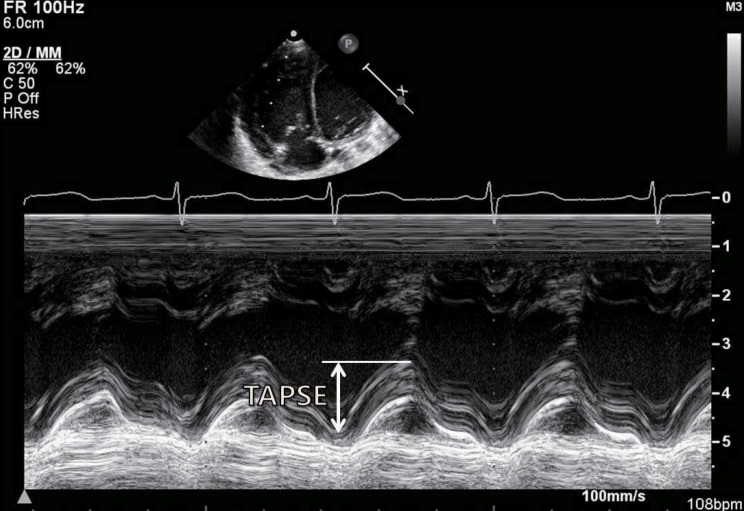
Measurements of the tricuspid annular plane systolic excursion (TAPSE) using M-mode echocardiography at the junction of the tricuspid valve plane with the free wall of the right ventricle.
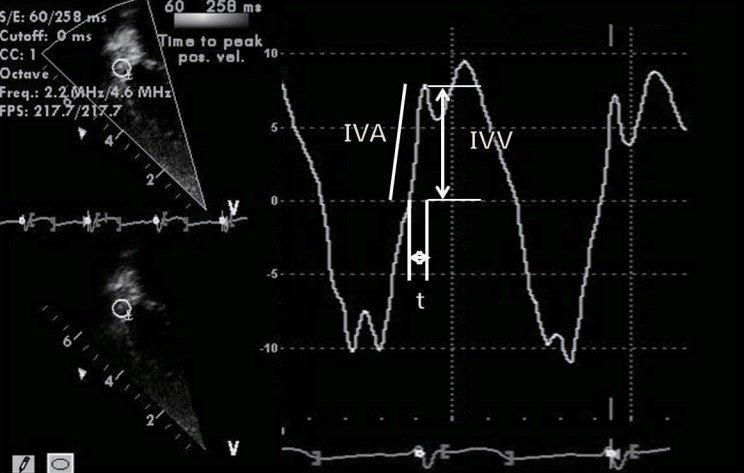
Measurement of isovolumic acceleration (IVA) during isovolumic contraction at the level of the tricuspid annulus using a tissue Doppler echocardiography spectral curve. IVV, peak isovolumic velocity; t, time from zero crossing to peak isovolumic velocity. IVA=IVV/t.
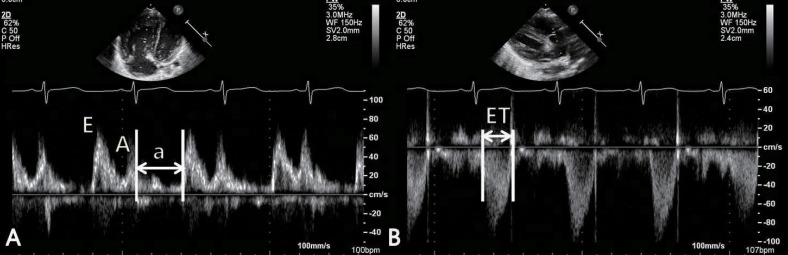
Measurement of right ventricular (RV) myocardial performance index (MPI) using pulsed-wave Doppler at the tip of tricuspid leaflets in the apical 4-chamber view (A) and at the site of just below the pulmonary valve in the RV outflow tract view (B). E, rapid filling velocity; A, atrial filling velocity; a, sum of isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT) and ejection time (ET). RV MPI=(IVRT+IVCT)/ET=(a-ET)/ET.
Management of RV failure
For the management of RV failure, the cause and setting of the failure should be considered18). The ultimate treatment goals include optimization of preload, afterload, and contractility18). Maintenance of sinus rhythm and atrioventricular synchrony is especially important in RV failure because atrial fibrillation and atrioventricular block may have profound hemodynamic effects18). In patients with RV dysfunction and valvular heart disease or CHD, corrective surgery or percutaneous intervention should be considered in suitable candidates18,39,40). Clinically, the assessment of optimal preload in RV failure remains challenging18). If no hemodynamic improvement is observed with an initial fluid challenge of normal saline, volume loading should not be continued18). The hemodynamic improvement seen with nitric oxide is most likely secondary to selective pulmonary vasodilatation, resulting in a reduction in RV afterload and subsequent improvement in RV performance41). Acute responsiveness to pulmonary vasodilators is associated with a better prognosis and survival in patients with advanced heart failure42,43). In patients with acute hemodynamically compromising RV failure, inotropic or vasopressor support may be required18). Dobutamine is the most commonly used inotrope in cases of RV failure44). In patients with pulmonary hypertension, dobutamine at doses of 2-5 µg · kg-1 · min-1 increases cardiac output but decreases PVR44). The combination of dobutamine and nitric oxide in patients with pulmonary hypertension also has been shown to be beneficial44). Dopamine is used in severely hypotensive patients, whereas milrinone is preferred in the presence of tachyarrhythmias induced by dopamine or in patients on β-blockers18). Digoxin therapy for RV failure has been studied in pulmonary hypertension and chronic pulmonary disease18). Maintenance of sinus rhythm and heart rate control are important in RV failure. High-degree AV block or atrial fibrillation may have profound hemodynamic consequences18). The RVEF in patients with either systemic or pulmonic RV was improved by cardiac resynchronization therapy45). The effects of β-blockade and angiotensin-converting enzyme inhibition have been studied mainly in LV heart failure. In patients with biventricular failure, angiotensin-converting enzyme inhibition has been shown to increase RVEF and to reduce RV end-diastolic volume and filling pressures46). Small studies also have demonstrated that β-blockade with carvedilol or bisoprolol improves RV systolic function47). Clinical studies that assessed the role of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in the systemic right ventricle found that they do not improve exercise capacity or hemodynamics, although those studies may have been underpowered48). In patients with acute RV failure that is refractory to medical treatment, mechanical support with an right ventricle assisting device may be used as a bridge to transplantation or recovery18). The development of new therapeutic strategies for RV failure is warranted. These new strategies might include cell-based or gene therapies, new drugs, or new combinations of existing drugs2).
Conclusions
RV failure remains an important cause of morbidity and mortality in patients with CHD both before and after cardiac surgery or intervention. Although recent advances in imaging including cardiac MRI, echocardiography remains pivotal for the noninvasive assessment of the right ventricle. A comprehensive evaluation of RV function may improve risk assessment and lead to early and optimal management of RV failure in patients with CHD.
Notes
No potential conflict of interest relevant to this article was reported.