Clinical characteristics of Kawasaki disease with sterile pyuria
Article information
Abstract
Purpose
Kawasaki disease (KD) is a systemic vasculitis and affects many organ systems. It often presents sterile pyuria, microscopic hematuria, and proteinuria due to renal involvement. The aims of this study were to define clinical characteristics of acute KD patients with pyuria and to analyze meaning of pyuria in KD.
Methods
The medical records and laboratory findings including serum and urine test of 133 patients with KD admitted to Yeungnam University Hospital from March 2006 to December 2010 were reviewed retrospectively.
Results
Forty patients had sterile pyuria and their clinical characteristics including age, gender and body weight were not significantly different with those who did not have pyuria. Fever duration after treatment was significantly longer in KD patients with pyuria. Erythrocyte sedimentation rate, C-reactive protein and serum concentration of alanine aminotransferase were significantly higher in patients with pyuria. Hyponatremia and coronary artery lesion were seen more often in patients with pyuria but there was no significant difference. Also serum blood urea nitrogen was significantly higher in KD patients with pyuria. Urine β2-microglobulin was elevated in both patients groups and showed no difference between two groups.
Conclusion
We found more severe inflammatory reaction in KD patients with pyuria. We also found elevation of some useful parameters like β2-microglobulin that indicate renal involvement of KD through the urine test. Careful management and follow up will need for KD patients with pyuria and it is necessary in the future to study the specific parameters for renal involvement of KD.
Introduction
Kawasaki disease is an acute febrile disease frequently occurring mainly in infants and toddlers under the age of 5. This syndrome is characterized by a persistent fever and is not responsive to antibiotics for more than 5 days. Symptoms include polymorphous skin rash, nonpurulent conjunctivitis, inflammation of lips and the mouth, swollen hands and feet and cervical lymphadenopathy1). Since Tomisaku Kawasaki first described 50 cases in Japan in 1967, numerous cases have been report ed but the exact cause and pathogenesis are not completely known yet2-4).
Kawasaki disease is a systemic vasculitis causing acute inflammation by invading mainly medium sized blood vessels3) and may cause various symptoms by invading multiple organs. The findings accompanied by inflammation of the renal urinary tract are sterile pyuria, hematuria and proteinuria. It has been reported that the most common finding is sterile pyuria and accounts for 33% to 63% of total patients with Kawasaki diseases5-8). Many studies about parenchymal invasion accompanied in Kawasaki disease have been conducted. It has been reported that interstitial nephritis9-12), acute renal failure13-15), hemolytic uremic syndrome16,17), and nephrotic syndrome18) occurred in Kawasaki disease. In this study, we analyzed the implications of pyuria in the Kawasaki disease.
Materials and methods
One hundred thirty-three patients (male 82, female 51) who were diagnosed and treated with Kawasaki disease were divided into a group of pyuria and a control group, and the comparative analysis on clinical characteristics and test results of each group was conducted.
Medical records of Kawasaki disease from March 2006 to December 2010 at the Department of Pediatrics at Yeungnam University Hospital were compared and analyzed retrospectively. The diagnosis of Kawasaki disease was based on the criteria published by the American Heart Association in 200419). In addition to that, even if someone did not meet the criteria, if Kawasaki disease was suspected clinically and other similar diseases were excluded, he or she was diagnosed with atypical Kawasaki disease.
Blood and urine samples were collected from all patients in the acute phase admitted and the transthoracic echocardiography was conducted. Blood and urine were collected before using high-dose gamma globulin and aspirin. With the cooperation of infant patients, mid-stream urine was collected. Without the cooperation, urine was collected from infant patients using a sterile urine collecting bag.
Case having five or more white blood cells at high magnification on micro urine microscopy was defined as pyuria20). Once all patients were divided into the group of pyuria with five or more white blood cells and the control group with less than 5, their gender, age and weight were compared. The total number of white blood cells, blood pigment, number of platelet, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), sodium, potassium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, total protein and albumin, and estimated creatinine clearance were measured through blood tests. Microscopic hematuria (five or more red blood cells on micro urine microscopy), β2-microglobulin (β2-MG) and total proteins were measured through urine collection. Estimated creatinine clearance was calculated with the Schwartz formula which is being used primarily in children21). Blood and urine culture tests were conducted in all patients. If bacteria were homogenous in culture tests, it was excluded in this study.
We needed to determine whether or not the coronary arteries was normal based on the criteria published by Japan Kawasaki Disease Research Committee22). If it is observed that the internal diameter of the coronary arteries in toddlers of five years old or less was over 3 mm or it was more than the diameter of adjacent vessels, it was defined as the dilatation of coronary arteries. If it was over 8 mm, it was classified as a giant aneurysm.
Right after the diagnosis was made, a high-dose of immunoglobulin (2 g/kg) was intravenously injected for over 12 hours and a high-dose of aspirin (50 to 100 mg/kg) was administered to all patients at the same time. The antibiotics were not used in all patients. Once 24 hours had passed after the fever subsided, a low-dose of aspirin (3 to 5 mg/kg) was administered for up to three months which was considered the recovery period. We considered the response of treatment with duration of fever after treatment, coronary artery lesion and need of high-dose intravenous immunoglobulin retreatment.
Two groups were compared using the chi-square test and Student's t-test with IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) for statistics. All values with P<0.05 were considered statistically significant.
Results
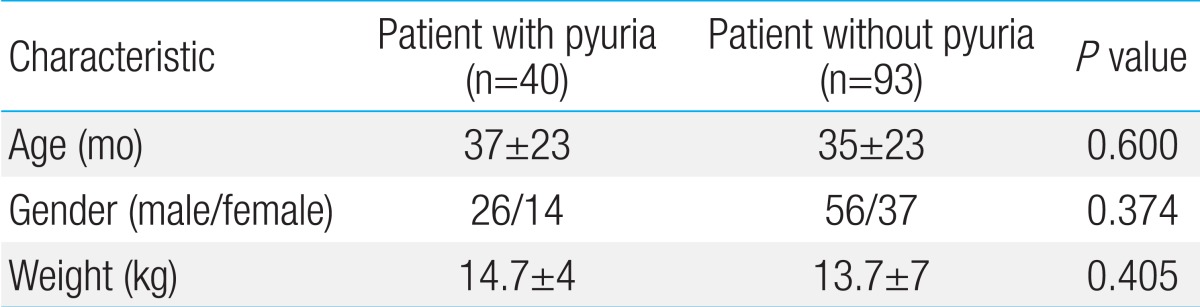
The pyuria group contained 40 patients (male 26, female 14) and accounted for 30.1% of total patients. The ratio of male to female was 1.9:1 and was not significant (P=0.374). In terms of the age of patients, the average age in the pyuria group was 38 months (range, 3 to 85 months) and the average age in the control group was 35.7 months (range, 3 to 130 months). There was no significant difference (P=0.600) (Table 1).
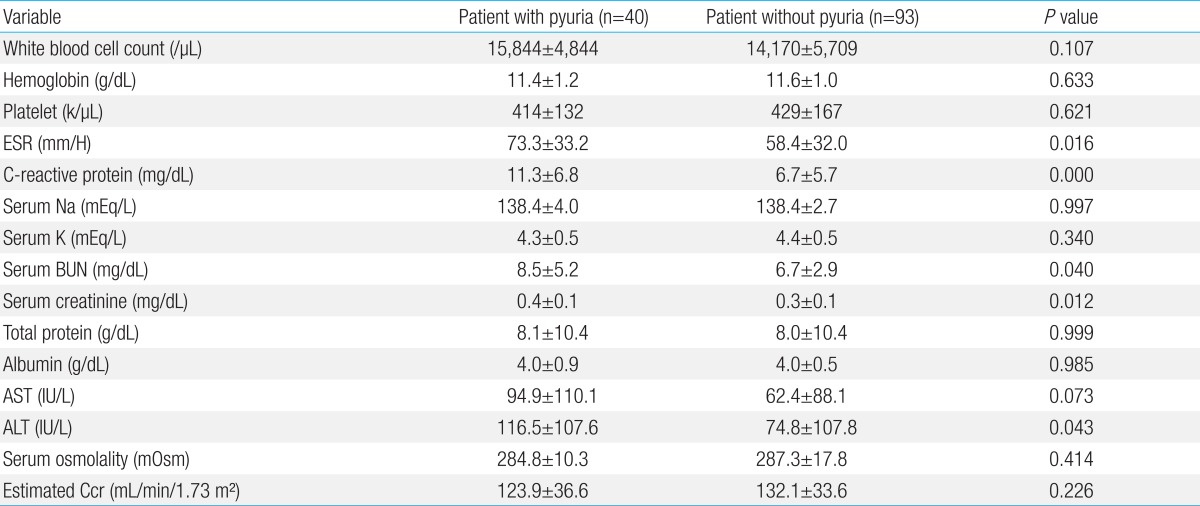
As shown in Table 2, the number of white blood cells did not show any significant difference through the blood tests (P=0.107). However, the ESR and CRP were significantly high in the pyuria group (P=0.016, and 0.000, respectively). There was no difference between the two groups in serum sodium, potassium, total protein, albumin, B-type natriuretic peptide, AST, and osmotic pressure (P>0.05), but ALT was significantly higher in the group of pyuria (P=0.043). The creatinine clearance in pyuria group was 123.9±36.6 mL/min/1.73 m2 and was 132.1±33.6 mL/min/1.73 m2 in control group. There was no difference between the two groups (P=0.226) (Table 2).
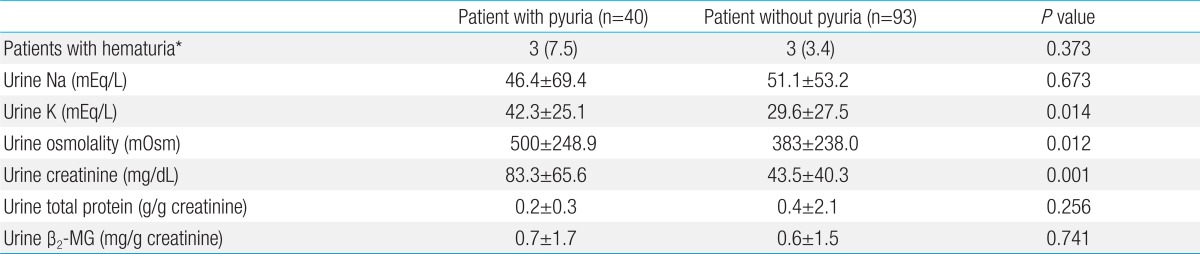
Urine tests showed a total of six patients (the pyuria group had 3 patients and the control group had 3 patients) who had microscopic hematuria. However, it was not a statistically significant difference due to the low number of patients. The pyuria group showed the significantly high values in urine osmolality, potassium and creatinine (P=0.012, P=0.014, and P=0.001, respectively). However, total protein and the ratio of creatinine to total proteins in urine showed no significant difference between the two groups (P=0.256). The ratio of urine β2-MG calibrated by creatinine was 0.77±1.7 mg/g creatinine in the pyuria group and 0.63±1.5 mg/g creatinine in the control group (P=0.741) (Table 3).
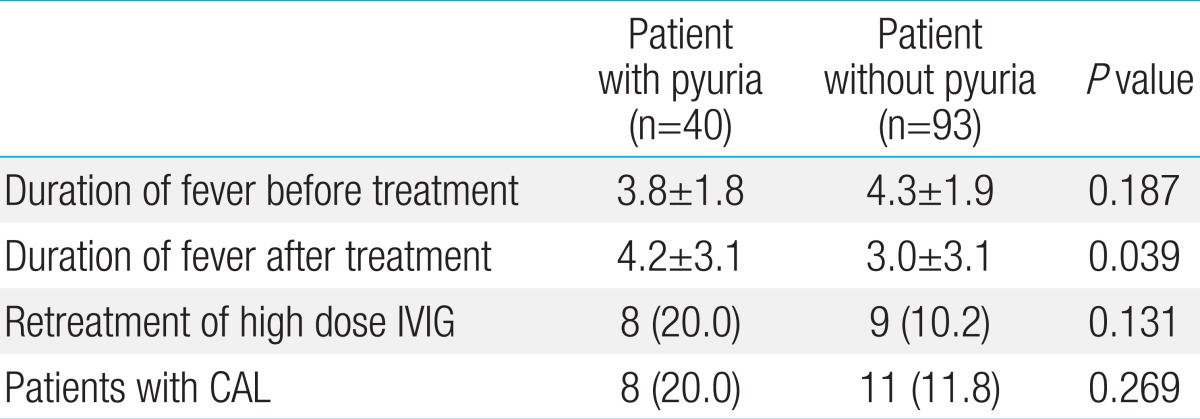
In the case of showing the abnormality of coronary arteries on transthoracic echocardiography, the pyuria group contained 8 patients (20%) and the control group contained 11 patients (11.8%). There was no statistically significant difference (P=0.269). The case of the giant aneurysm in which coronary arteries dilated to over 8 mm was not shown in the two groups (Table 4).
There was no statistically significant difference in the duration of fever before treatment between two groups (P=0.187). The duration of the fever after treatments on the acute phase was an average of 4.2 days in the pyuria group and an average of 3 days in the control group. It confirmed that the response to treatment was slower in the pyuria group compared to the control group (P=0.039) (Table 4).
Discussion
Kawasaki disease is a systemic vasculitis which frequently occurs mainly in infants and toddlers 5 years old or less may show the various symptoms and findings by involving multiple organs. In general, abnormal findings on urine tests which occur due to the invasion into the renal urinary tract include sterile pyuria, hematuria and proteinuria. The most common finding is sterile pyuria. However, not many studies about the meaning of pyuria in Kawasaki disease have been conducted. In this study, patients with Kawasaki disease who received treatments are divided into two groups such as the pyuria group which showed pyuria on urine test upon admission to the hospital and the control group. Comparative analysis on clinical progress characteristics, response to treatment, blood tests and urine tests are conducted to identify the meaning of pyuria in the Kawasaki disease.
Therapy for the acute phase was carried out using high-dose immunoglobulin and high-dose aspirin therapy. The number of patients who were treated with high-dose immunoglobulin more than twice was eight in the pyuria group and nine in the control group. There was no significant difference (P=0.131). The duration of fever after the treatments on the acute phase showed that the response to treatment for the acute phase was slower in the pyuria group compared to the control group (P=0.039). In addition, ESR and CRP were significantly high in the pyuria group (P<0.001). The abnormality in liver function is sometimes mentioned as an indicator for the systemic inflammatory response of Kawasaki disease22). In this study, the ALT of pyuria group was significantly higher than the control group. However, the AST did not show any significant differences.
All of these results showed that the inflammation response in patients with Kawasaki disease in the acute phase accompanying pyuria was more severe. The response to antiinflammatory treatment thus was considered slow. On this basis, we think that the presence of pyuria with Kawasaki disease in the acute phase reflects the severity of inflammation.
Currently, many studies about the invasion into renal urinary tracts in Kawasaki disease have been underway. First of all, in order to prove the invasion into renal urinary tract in Kawasaki disease, we need to check the renal function of patients with Kawasaki disease. For this, we need to measure the accurate glomerular filtration rate. By far the most commonly used methods are to calculate the glomerular filtration rate by measuring creatinine in blood and urine, and urine volume. However, since creatinine is influenced by age, muscle mass and food intake, it has a disadvantage to reflect the glomerular filtration rate inaccurately. Therefore, there are continuing debates about other indicators to measure the glomerular filtration23,24). Cystatin-C (Cys-C) is widely being used now as an indicator to reflect the renal function relatively accurate without the limitation of age, muscle mass, and protein intakes compared to creatinine25). However, since it has been known that Cys-C is also affected by body composition26) and the increase of CRP327), debates are still going on. Other indicators which are being studied include neutrophil gelatinase associated lipocalin28,29) and kidney injury molecule-130). Although it is known that these methods sensitively predict the renal deterioration in a blood and urine test, they are less cost effective.
β2-MG which was used in this study has the implication in both blood and urine tests. Since it is a small globular protein with the molecular weight of 11,800 Da, it can pass relatively freely through the renal glomerular basement membrane and the most of them are absorbed through the proximal tubule. Thus, only a small portion is excreted if renal tubular function is normal31,32). Since β2-MG in urine can be measured with noninvasive and easy methods, it can be used to evaluate the maturation of the renal tubule in infants33). In case of a particular nephrotoxicity, the faster abnormal findings are shown in nephrotoxicity caused by antibiotics of aminoglycosides compared to serum creatinine34). It is being used as a useful indicator to check the function of renal urinary tract. Ohta et al.35) reported the possibility of renal involvement by the increase of interleukin-6, N-acetyl-β-D-glucosaminidase in urine of patients with Kawasaki disease. In this study, urinary β2-MG which is calibrated by urinary creatine are high in both groups. This is the result with which we can infer the damage on renal function in most patients with Kawasaki disease.
Elevated levels of both BUN and creatinine in blood can be used as another indicator to imply the significant damage on renal function, but the elevated level of both BUN and creatinine were not observed in this study. BUN was higher in the pyuria group. It may not be due to the damage on renal function but it is thought to be caused by dehydration due to high fever and anorexia. In addition, in order to determine the presence or absence of proteinuria, a urinary stick test and the ratio of protein and creatinine in urine were measured, but there was no significant difference between the two groups. Schwartz formula21) which is widely used for children is used to calculate creatinine clearance. It was also no difference between the two groups.
Besides blood and urine tests, there are some reports to confirm the invasion of renal parenchyme in Kawasaki disease through radiological tests. Nardi et al.13) conducted the renal ultrasonography on four patients out of seven with Kawasaki disease showing hematuria, proteinuria and pyuria and confirmed the findings of increased cortical echogenicity, enlarged kidney and enhanced corticomedullary differentiation. Wang et al.36) conducted technetium-99m-dimercaptosuccinic acid renal scintigraphy single photon emission computed tomography and confirmed the renal scarring caused by inflammation in 26 patients (52%) out of 50 patients with Kawasaki disease. In this study, radiological tests were not added, but we could estimate the decrease of renal function due to the increase of urinary β2-MG and we need to develop a more desirable indicators to replace the creatinine in blood and urine in order to determine the renal involvement of Kawasaki disease.
In conclusion, we found that patients with Kawasaki disease with pyuria have more severe inflammatory response in the acute phase and the therapeutic response is slower as well. Therefore, more careful observations and tracking the clinical progress including complications caused by invasion of renal parenchyma in patients with Kawasaki disease and the additional research will be needed in the future.