Stroke after percutaneous transhepatic variceal obliteration of esophageal varix in Caroli syndrome
Article information
Abstract
Here we present the case of an 11-year-old female patient diagnosed with Caroli syndrome, who had refractory esophageal varices. The patient had a history of recurrent bleeding from esophageal varices, which was treated with endoscopic variceal ligation thrice over a period of 2 years. However, the bleeding was not controlled. When the patient finally visited the Emergency Department, the hemoglobin level was 4.4 g/dL. Transhepatic intrajugular portosystemic shunt was unsuccessful. Subsequently, the patient underwent percutaneous transhepatic variceal obliteration. Twenty hours after this procedure, the patient complained of aphasia, dizziness, headache, and general weakness. Six hours later, the patient became drowsy and unresponsive to painful stimuli. Lipiodol particles used to embolize the coronary and posterior gastric veins might have passed into the systemic arterial circulation, and they were found to be lodged in the brain, kidney, lung, and stomach. There was no abnormality of the portal vein on portal venography, and blood flow to the azygos vein through the paravertebral and hemiazygos systems was found to drain to the systemic circulation on coronary venography. Contrast echocardiography showed no pulmonary arteriovenous fistula. Symptoms improved with conservative management, and the esophageal varices were found to have improved on esophagogastroduodenoscopy.
Introduction
Percutaneous transhepatic variceal obliteration (PTVO) was introduced in 1974 as a treatment for bleeding esophageal varices can be used for patients who continue to bleed despite of medical therapy for inoperable recurrent variceal bleeding1). Even though PTVO was proven to control the acute variceal bleeding, several clinical questions have not been fully resolved and numerous complications have been recognized2). We report a case of stroke by systemic emboli after injection of embolization agents used for obliteration of esophageal varix, in the absence of evidence of right to left shunt. The female 11-year-old patient has recovered from the stroke and has been doing well without neurologic symptoms.
Case report
A 11-year-old girl with Calori syndrome was admitted for PTVO because of recurrent esophageal variceal bleeding in spite of conventional treatments. Three years previous, the patient presented with hematemesis, and visited our Emergency Department. On physical examination, the patient was pale and the abdomen was protuberant with a palpable and firm spleen below the left costal margin. Blood abnormalities were as follows: platelet count, 53,000/µL; hemoblogin, 8.5 g/dL; international normalized ratio of prothrombin time, 1.25; aspartate transaminase/alanine transaminase, 24/11 IU/L; total bilirubin, 0.4 mg/dL; albumin, 3.6 g/dL; alkaline phosphatase, 110 IU/L; γ-glutamyl transpeptidase, 13 IU/L; and creatinine, 0.39 mg/dL. An abdominal computed tomography showed diffuse bile duct dilatation with liver cirrhosis and bilateral cystic renal disease. Ultrasonograhy-guided liver biopsy revealed congenital hepatic fibrosis. The patient was diagnosed with Caroli syndrome. Polycystic kidney and hepatic disease 1 gene (PKHD1) was not detected. Grade III esophageal varices with red wale sign at distal and midesophagus and portal hypertensive gastropathy were observed on esophagogastroduodenoscopy. Endoscopic variceal ligation (EVL) was performed three times over the following 2 years.
Six months later, the patient was readmitted for severe recurrent bleeding. Endoscopy demonstrated ruptured esophageal varices and portal hypertensive gastropathy. The hemoglobin level was 4.4 g/dL, and three transfusions of packed red blood cells were necessary to restore the hemoglobin level to 11.6 g/dL. However, the varices continued to bleed. Medical therapy including octreotide and propranolol was unable to stop the bleeding. Transjugular intrahepatic portosystemic shunt (TIPS) was performed but it also failed to control bleeding because of the technical difficulties. Therefore, PTVO was suggested as an option by a radiologic interventionist wihin 50 minites. Under general anesthesia, portal vein branch was punctured by chiba needle. On splenoportography, porterior enlarged gastric vein and coronary vein were observed, blood flows through gastric veins and esophageal veins were found. Embolization for gastroesophageal varix from coronary and posterior gastric vein was performed. An emulsion was made with glue and lipiodol in a 1:16 ratio (Fig. 1). On venography, blood outflow from gastric varix and esophageal varix was detected, but other collateral circulations connecting directly to systemic circulation such as inferior vena cava was not detected. A small amount of emulsion was drawn out to both pulmonary arteries when glue embolization approached the posterior gastric vein. Close observation was required. On coronary venography, blood flow from esophageal varix and azygous vein through paravertebral and hemiazygous system were observed. This blood flow was drained to systemic circulation, so embolization was performed with emulsion made with glue and lipiodol in a 1:7 ratio. On splenoportography after embolization, gastroesophageal varix was obliterated completely, portal vein showed no abnormality. At that time, the patient did not show any symptoms and signs.

(A) Angiographic portography revealed portal vein, coronary vein, posterior gastric vein, and esophageal varices. (B) Venography showed complete occlusion of the varices after injection of contrast embolic material.
Twenty hours after PTVO, the patient complained of aphasia, dizziness, headache and upper and lower extremities motor and sensory weakness. Six hours later, the patient became drowsy and unresponsive to the painful stimuli. Her upper muscle power was symmetric, she could not able to maintain position against slight resistance or gravity (grade III). Her lower muscle power was symmetric, she could not maintain against moderate resistance (grade IV). Both pupillary reflex was present, pupils were isocoric with 4 mm. But, the patient's respiratory pattern remained stable. Arterial blood gas analysis showed normal. On X-rays, small linear or dot-like opacities appeared in the lung fields as well as in the abdomen. On brain computed tomography scan, multifocal high-density lesions in the parasagittal areas of cerebral hemisphere and cerebellum were found (Fig. 2). On abdominal computed tomography scan, systemic embolization of lipiodol involving lung, kidney, liver, and stomach was observed, pulmonary edema was suspected as well. Laboratory results were as follows: platelet count, 53000/µL; hemoblogin, 10.5 g/dL; international normalized ratio of prothrombin time, 1.04; aspartate transaminase/alanine transaminase, 35/18 IU/L; total bilirubin, 0.9 mg/dL; albumin, 4.1 g/dL; blood urea nitrogen, 5.2 mg/dL; and creatinine, 0.37 mg/dL. Urine analysis revealed no abnormal finding.

Brain computed tomography revealed multifocal high-density lesions in the parasagittal areas of the cerebral hemisphere (large arrow), cerebellum (arrowhead), occipital lobe (small arrow), caudate nucleus, and thalamus.
Contrast echocardiography was performed twice, which did not reveal the evidence of a right-to-left shunt, except for a nonfunctioning patent foramen ovale. Conservative management including rehabilitation therapy resulted in the steady improvement in the symptoms over 20 days. A follow-up endoscopy revealed improved esophageal varices and portal hypertensive gastropathy (Fig. 3). The opacities on chest X-ray ameliorated considerably (Fig. 4). One month later, the patient remained well, and there was no further neurologic abnormality.
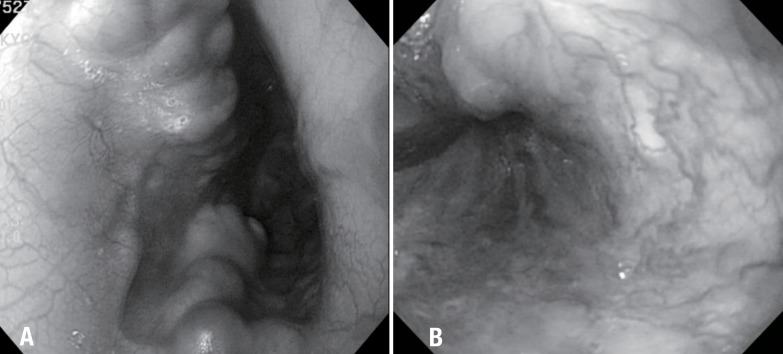
On esophagogastroduodenostopy, (A) grade III varices with red wale sign were found at the distal esophagus and (B) the varices improved 5 months after percutaneous transhepatic variceal obliteration.
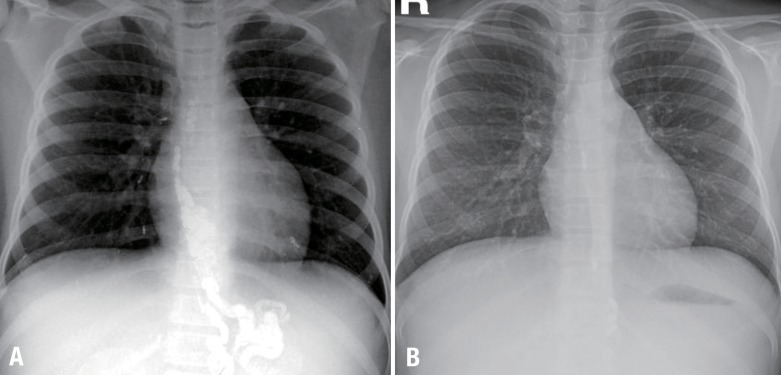
(A) Simple chest radiolography revealed small linear or dot-like opacities in the lung fields and the abdomen. (B) The opacities reduced considerably 1 month after percutaneous transhepatic variceal obliteration. No radiopaque embolic material was apparent on the gastroesophageal varices. The disappearance of multiple small linear or dot-like opacities in both lung fields was evident.
Discussion
Caroli disease is a rare congenital disorder characterized by segmental, nonobstructive dilatation of intrahepatic bile ducts. The term Caroli syndrome is used for the association of Caroli disease with congenital hepatic fibrosis, which is characterized by the maldevelopment of small-sized interlobular bile duct3,4). The clinical manifestations of Caroli syndrome depend on the more predominant pathologic lesion between ectasia of the intrahepatic ducts and hepatic fibrosis. The signs and symptoms are mainly those of portal hypertension (splenomegaly and variceal bleeding). There may be evolution into true liver cirrhosis5).
Approximately half of the patients with cirrhosis may develop esophageal varices6), and two-thirds of those may eventually show hemorrhage from varices7). The mortality rate associated with esophageal bleeding is highest during the episode of acute bleeding. When the varices remain untreated after recovery from acute bleeding, a 95% incidence of recurrent bleeding and death within 2 to 5 years in 90% to 100% of the patients has been reported8). The goal in the treatment of bleeding varices is to stop active bleeding and to prevent recurrent bleeding. Combination therapy including the early use of portal pressure-reducing drugs such as somatostatin, octreotide, and terlipressin, and EVL has become the first-line treatment in the management of acute variceal bleeding9). Since its first introduction in the 1980s, TIPS has played an increasingly important role in the management and treatment of the complications of portal hypertension. In 2005, the American Association for the Study of Liver Diseases published the practice guidelines for the use of TIPS in the management of portal hypertension10).
The invasive but nonsurgical modality of PTVO of the coronary and short gastric veins as treatment for bleeding esophageal varices was introduced in 19741). It may be considered in patients who continue to bleed despite of medical therapy, and in inoperable patients with recurrent bleeding. Benner et al.2) reported complications of PTVO. According to their report, major complications were intraperitoneal bleeding, hemopneumothorax, bleeding from an intercostal artery, hepatic laceration and portal vein thrombosis. Minor complications were pleural effusion, partial portal vein thrombus, penetration of biliary tract, ascites leak, transient hypotension and intimal damage of splenic vein. Intrahepatic arteriovenous fistula occurred in 26.2% of the cases after percutaneous transhepatic catheterization of the portal venous system11).
Systemic emboli have been reported previously following PTVO. One patient who had a stroke 8 hours after the procedure and died 3 hours later. At autopsy, gelfoam emboli were found in the lungs and the brain. The only pertinent autopsy finding was an asymptomatic patent foramen ovale12). There are other 2 cases of cerebral stroke in cirrhotic patients following endoscopic obturation of esophageal varices with isobutyl-2-Cyanoacrylate. In both cases, hemiplagia appeared several hours after the procedure. A brain computed tomographic scan showed radiodense material in the cerebral arteries duo to dissemination of isobutyl-2-Cyanoacrylate. One patient died, the other improved slowly.13) Ellman et al.14) reported two cases in which particles of gelfoam used to embolize the coronary and short gastric veins passed into the systemic arterial circulation through a porto-pulmonary anastomosis and embolized in the brain, spleen and heart. Patients with cirrhosis and portal hypertension can demonstrate a right-to-left shunt, caused by anastomosis connecting the periesophageal veins with pulmonary veins. When portal venous pressure rises, a number of natural portosystemic anastomoses enlarge to allow the high-pressure portal circulation to enter the low-pressure systemic venous circulation. These include the short gastric veins, which anastomoses with the azygos and hemiazygos systems around the esophagus, and the coronary veins, which may communicate directly with the inferior vena cava or left portal vein. Subclinical portopulmonary anastomosis may be a cause of serious systemic embolic complications during the embolization procedure for esophageal varices15). Three patients with spontaneous porto-pulmonary shunts in the cirrhosis associated with the embolization of gelfoam during the treatment of esophageal varices, resulting in strokes16). In our case, however, other collateral circulation connecting directly to systemic circulation, such as inferior vena cava, was not detected. There was no abnormality of the portal vein on portal venography and blood flow to azygos vein through paravertebral and the hemiazygous system drained to systemic circulation on coronary venogram. Emboli were disseminated to kidney, stomach, liver, spleen as well as lung. Therefore, porto-systemic anastomoses could be excluded.
Contrast echocardiography is more sensitive than any other diagnostic tools such as portography for porto-pulmonary anastomosis. When performing contrast echocardiography by the portal approach, the following three main routes might produce contrast echos in the left heart: porto-pulmonary anastomosis, intracardiac right-to-left shunt and intrapulmonary right-to-left shunt15). In our patient, contrast echocardiography was performed using peripherally injected saline to produce microbubbles that opacify the right atrium. Microbubbles were not seen in the left heart. Therefore, we were able to rule out the possibility of the above-mentioned three shunts.
In our case, different hypotheses may be raised, systemic emboli via unconfirmed portopulmonary venous shunt despite of close work up and injection into the arterial circulation. But, we could not find out exact etiology of the complication.
As lipiodol is a lipid-soluble agent and can disappear by phagocytosis, it seems that the symptoms of the patient would not progress further, and rather, they ameliorated. Multiple emboli to the heart or brain could have serious consequences, but fortunately, this patient recovered completely from neurologic damages. The patient has been doing well and has followed at outpatient clinic at regular intervals. PTVO is useful in stopping acute variceal bleeding but we have to keep in mind the possibility of the occurrence of complication.
Notes
No potential conflict of interest relevant to this article was reported.