High-dose chemotherapy and autologous peripheral blood stem cell transplantation in the treatment of children and adolescents with Ewing sarcoma family of tumors
Article information
Abstract
Purpose
We performed a pilot study to determine the benefit of high-dose chemotherapy and autologous peripheral blood stem cell transplantation (HDCT/autoPBSCT) for patients with Ewing sarcoma family of tumors.
Methods
We retrospectively analyzed the data of patients who received HDCT/autoPBSCT at Korea Cancer Center Hospital. Patients with relapsed, metastatic, or centrally located tumors were eligible for the study.
Results
A total of 9 patients (3 male, 6 female), with a median age at HDCT/autoPBSCT of 13.4 years (range, 7.1 to 28.2 years), were included in this study. Patients underwent conventional chemotherapy and local control either by surgery or radiation therapy, and had achieved complete response (CR, n=7), partial response (n=1), or stable disease (n=1) prior to HDCT/autoPBSCT. There was no transplant-related mortality. However, the median duration of overall survival and event-free survival after HDCT/autoPBSCT were 13.3 months (range, 5.3 to 44.5 months) and 6.2 months (range, 2.1 to 44.5 months), respectively. At present, 4 patients are alive and 5 patients who experienced adverse events (2 metastasis, 2 local recur, and 1 progressive disease) survived for a median time of 2.8 months (range, 0.1 to 10.7 months). The 2-year survival after HDCT/autoPBSCT was 44.4%±16.6% and disease status at the time of HDCT/autoPBSCT tended to influence survival (57.1%±18.7% of cases with CR vs. 0% of cases with non-CR, P=0.07).
Conclusion
Disease status at HDCT/autoPBSCT tended to influence survival. Further studies are necessary to define the role of HDCT/autoPBSCT and to identify subgroup of patients who might benefit from this investigational treatment.
Introduction
Combination chemotherapy significantly improved the outcome of patients with Ewing sarcoma family of tumors (ESFT)1). However, patients with high-risk features, i.e., metastasis at presentation or recurrent disease, still have a very poor prognosis, with a survival rate of 7.30%1). It has been suggested that patients with poor prognostic features have chemosensitivie tumors2). In vitro data showed a positive dose-response relationship for ESFT cells treated with alkylating agents3), platinum agents, and epipodophyllotoxins4). Myelosuppression is the dose-limiting toxicity for all of these agents2,5,6). Stem cell transplantation could overcome this problem and allow dose escalation2,4,5). Several groups have previously evaluated the efficacy of high-dose chemotherapy (HDCT) and stem cell transplantation in ESFT patients, but the therapeutic efficacy of HDCT is still controversial2,4,5,7-15). Previous studies analyzed small numbers of cases with inhomogeneous clinical features and produced conflicting results, and there has been no randomized comparison between high-dose therapy and conventional-dose therapy.
Few data exist on the efficacy of HDCT in the treatment of Asian patients with ESFT. It has been suggested that incidence and outcome differences of ESFT have a genetic basis16). The incidence of ESFT is very low in South Korea, with fewer than 40 new cases diagnosed annually17). In countries where ESFT is rarely encountered, studies aimed at improving the survival of ESFT patients might be best approached using collaborative or multinational approaches, and the establishment of a retrospective data set is a prerequisite for such studies. This retrospective analysis was performed as a pilot study to evaluate the efficacy of HDCT and autologous peripheral blood stem cell transplantation (HDCT/autoPBSCT) for Korean patients with high-risk ESFT. We reviewed the data from children and adults with ESFT who received HDCT/autoPBSCT at the Korea Cancer Center Hospital, who had 1) recurrent tumors, 2) metastatic disease at the time of diagnosis, or 3) a centrally located tumor. We analyzed the clinicopathologic characteristics and treatment outcomes to identify a subgroup of patients who might benefit from this treatment.
Materials and methods
1. Patients
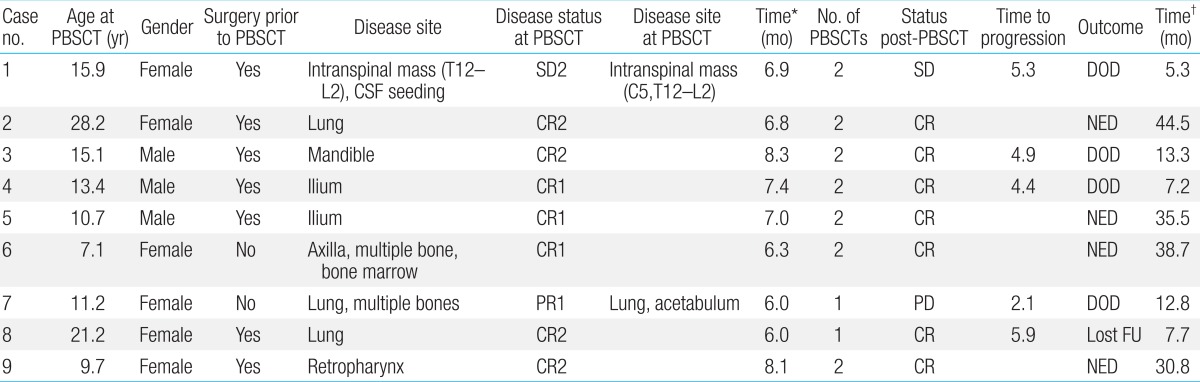
We retrospectively analyzed the data of 9 patients with ESFT who received HDCT/autoPBSCT between January 2007 and December 2010 at the Department of Pediatrics, Korea Cancer Center Hospital. Patients with ESFT were considered eligible for this therapy if they had multiple metastases at the time of diagnosis (n=2), centrally located tumors (n=2), or recurrent disease (n=5). Normal renal, cardiac, and hepatic functions were required. Prior to HDCT/autoPBSCT, patients underwent conventional chemotherapy and local control either by surgery or radiation therapy. Seven patients were treated with tandem HDCT/autoPBSCT. Detailed patient information is presented in Table 1.
2. Diagnosis and treatments prior to HDCT/autoPBSCT
ESFT was diagnosed by immunohistochemical methods, that is, the presence of small round cells, with no cytologic, histologic, or immunohistochemical feature of lymphoma, rhabdomyosarcoma, or neuroblastoma. It is recommended that the diagnosis of ESFT should be based on histologic and cytogenetic analyses, however, due to the retrospective nature of this study, cytogenetic analyses were not feasible. The 2 patients with relapsed ESFT first received surgery and then received radiation therapy and chemotherapy. The remaining 7 patients received neoadjuvant chemotherapy consisting of vincristine+ifosfami de+doxorubicin+etoposide (n=7) or vincristine+doxorubicin+c yclophosphamide alternating with ifosfamide+etoposide (n=1) or gemcitabine+docetaxel (n=1). Eight cases were eligible for response evaluation. The timing of surgery varied, and usually, patients underwent surgery to remove the primary tumor after receiving 4.6 courses of chemotherapy. Histologic response was evaluated in 4 cases and 3 cases showed good response (<10% residual viable tumor). The timing of leukapheresis varied depending on the patient's condition, and patients with pelvic or mandibular tumors underwent leukapheresis before definitive surgery. Four patients underwent 2 rounds of leukapheresis. Granulocyte-colony stimulating factor (G-CSF) (5.10 µg/kg) was subcutaneously administered if the neutrophil count fell below 0.5×109/L after chemotherapy, and G-CSF administration was continued until the completion of stem cell collection. Stem cell collection was started when the white blood cell count exceeded 1.0×109/L with a monocytosis after nadir. We aimed to collect a minimum of 2×106 CD34+ cells/kg, and ideally more than 5×106 CD34+ cells/kg, for rescue during the tandem HDCT with the peripheral blood stem cells (PBSCs) collected during a single leukapheresis round. If the collected number of CD34+ cells was not sufficient, another round of leukapheresis was performed.
3. HDCT/autoPBSCT
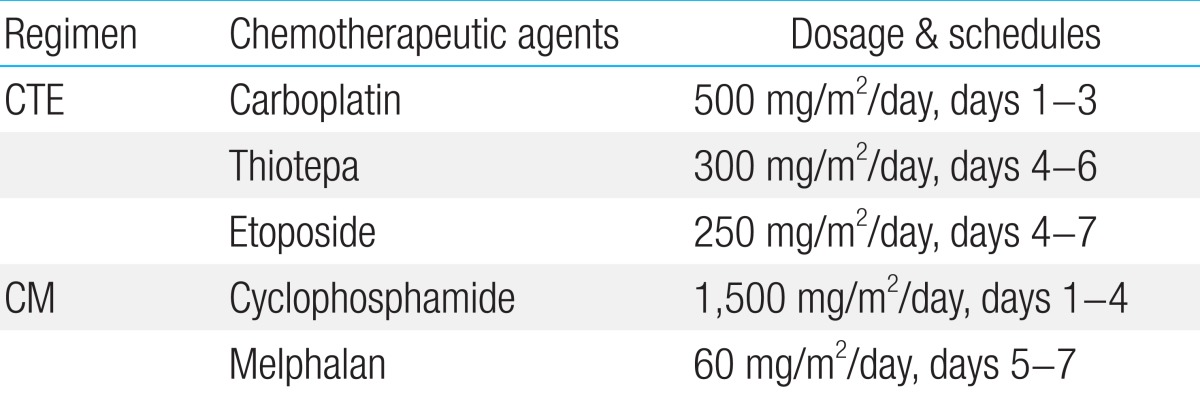
Patients underwent HDCT/autoPBSCT after completing conventional chemotherapy and local tumor control with either surgery or radiation therapy. The HDCT regimens used in our patients were; carboplatin+thiotepa+etoposide for the first HDCT, and cyclophosphamide+melphalan for the second HDCT (Table 2). PBSCs were infused 2 days after the end of HDCT, and the median CD34+ cell count was 3.8×106 cells/kg body weight (range, 2.2-6.0×106 cells/kg body weight).
4. Response evaluation
Clinical response was evaluated using either computed tomography (CT) or positron emission tomography-CT (PET-CT). Usually, the response of lung metastatic lesions was assessed radiologically by using CT, and response was defined according to the Response Evaluation Criteria In Solid Tumors (RECIST): complete response (CR), disappearance of all target lesions; partial response (PR), ≥30% decrease from baseline; progressive disease (PD), ≥20% increase over smallest sum observed or appearance of new lesions; and stable disease (SD), neither PR nor PD criteria met18). Bone metastatic lesions were monitored using [F-18]-fluorodeoxy-D-glucose (FDG)-PET and responses were recorded under the following categories: complete metabolic response, complete resolution of 18F-FDG uptake; partial metabolic response, a minimum reduction of 15%±25% in the tumor 18F-FDG standardized uptake value (SUV) after 1 cycle of chemotherapy and ≥25% reduction after more than 1 treatment cycle; stable metabolic disease, an increase in the tumor 18F-FDG SUV of <25% or a decrease of <15% and no visible increase in the extent of 18F-FDG tumor uptake; and progressive metabolic disease (PMD), an increase in the tumor 18F-FDG SUV of >25% within the tumor region defined on the baseline scan, a visible increase in the extent of 18F-FDG tumor uptake, or the appearance of new 18F-FDG uptake lesions18).
5. Toxicity
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (ver. 3.0).
6. Statistical analysis
Survival analysis was performed using the Kaplan-Meier method, from the date of the first HDCT/autoPBSCT. Events were defined as disease progression or any other cause of death.
Results
1. Status prior to and after HDCT/autoPBSCT
There were 6 CR and 3 PR after the mobilization chemotherapy (Table 1). RECIST was used as the response criteria for most of the patients, although case no. 6 was presumed to have metabolic CR. This patient showed very good response to both conventional and mobilization chemotherapy, with PR of the primary lesion (axilla) and CR of the metastatic lesions (bone and bone marrow). The response of her axillary tumor was classified as PR according to RECIST; however, the maximal SUV value decreased after 2 courses of mobilization chemotherapy from 2.1 to 1.7 and then to 0.9. Radiotherapy was administered after HDCT/autoPBSCT for local tumor control.
There were 7 CR, 1 PR, and 1 SD case at the time of HDCT/autoPBSCT. One patient with PR (patient no. 2) underwent surgery after mobilization chemotherapy, and subsequently achieved CR. For patient no.7, the primary lung tumor and one of the metastatic bone lesions (acetabulum) showed PR and the remaining multiple metastatic bone lesions showed CR at the time of HDCT/autoPBSCT. Patient no.1 developed a metastatic lesion 2.5 months after mobilization chemotherapy. She was treated with chemotherapy and radiotherapy and showed SD at the time of HDCT/autoPBSCT. After HDCT/autoPBSCT, 7 cases showed CR and 2 showed SD.
2. Toxicity and hematologic recovery after HDCT/autoPBSCT
All patients experienced grade 4 leukopenia, neutropenia, and thrombocytopenia after HDCT/autoPBSCT. Nonhematologic toxicities observed during the 16 courses of HDCT/autoPBSCT were as follows: grade 2/3 stomatitis (n=4), sepsis (n=3; 1 vancomycin-resistant enterococci, 1 Streptococcus oralis, and 1 unidentified pathogen), veno-occlusive disease (n=2), grade 2/3 diarrhea (n=2), herpes zoster (n=1), hemorrhagic cystitis (n=1), and pneumonia (n=1). There was no significant difference in nonhematologic toxicity between the first and second course of HDCT.
A median of 10 days (range, 9 to 14 days) was required to reach a neutrophil count greater than 0.5×109/L, a median of 11 days (range, 9 to 15 days), to reach a neutrophil count greater than 1.0×109/L, and a median of 24 days (range, 13 to 248 days), to reach a platelet count greater than 50×109/L. The median time for hematologic recovery was the same for the first and second course of HDCT/autoPBSCT.
3. Survival after HDCT/autoPBSCT
The median follow-up duration was 21.6 months (range, 12.2 to 51.3 months). From the day of the first HDCT/autoPBSCT, the median overall survival (OS) and event-free survival (EFS) were 13.3 months (range, 5.3 to 44.5 months) and 6.2 months (range, 2.1 to 44.5 months), respectively. Four patients were still alive after completion of the study. Five patients experienced disease adverse events (2 metastasis, 2 local recurrences, and 1 PD) at a median time of 4.9 months (range, 2.1 to 5.9 months), and of these, 3 received salvage chemotherapy consisting of vincristin e+topotecan+cyclophosphamide (n=1), gemcitabine+docetaxel (n=1), or cyclophosphamide+topotecan (n=1). However, all of them died after a median time of 2.8 months (range, 0.1 to 10.7 months). The 2-year OS and EFS after HDCT/autoPBSCT were identical (44.4%±16.6%). Disease status at the time of HDCT/autoPBSCT tended to influence survival (Fig. 1) (P=0.07). However, survival rates were not statistically different between 4 patients who received HDCT/autoPBSCT as an initial treatment and 5 patients who underwent HDCT/autoPBSCT after disease relapse (50.0%±25.0% vs. 40.0%±21.9%, P=0.81). Interestingly, the presence of metastasis at the time of diagnosis did not influence survival after HDCT/autoPBSCT (42.9%±18.7% vs. 50.0%±35.4%, P=0.76).
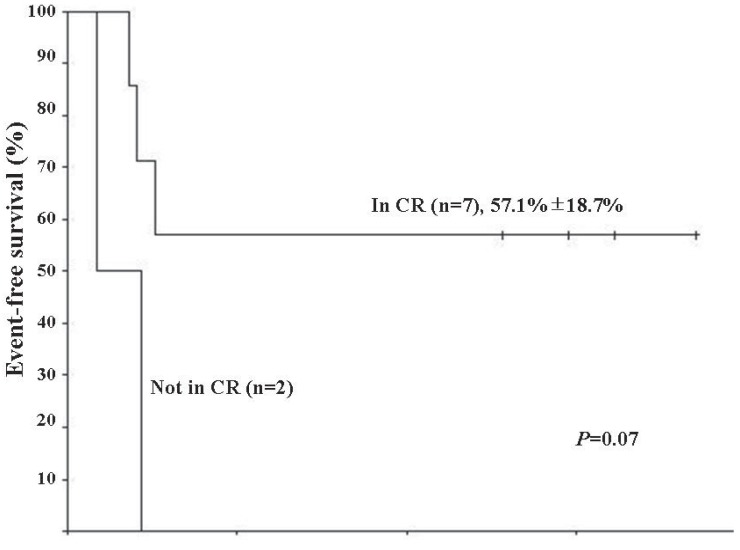
Kaplan-Meier estimate of event-free survival (EFS) in Ewing sarcoma family of tumors patients that underwent high-dose chemotherapy and autologous peripheral blood stem cell transplantation (HDCT/autoPBSCT). Patients who were in complete response (CR) at the time of HDCT/autoPBSCT tended to have better EFS than those who were not in CR (P=0.07).
Discussion
We analyzed the clinicopathologic characteristics and outcome of high-risk ESFT patients who received HDCT/autoPBSCT as a consolidating treatment. High-risk cases were defined as those involving metastatic disease at the time of diagnosis, recurrent disease, or tumors located in the central parts of the body. We observed that disease status at the time of HDCT/autoPBSCT tended to influence survival, although our findings still remain inconclusive regarding the benefit of HDCT/autoPBSCT for this subgroup of patients.
Some limitations of the present study must be considered. The incidence of ESFT is very low in Korea, and accordingly, our study cohort was composed of a very small number of cases. Furthermore, because of its retrospective nature, this study was not prospectively randomized and treatments before HDCT/autoPBSCT were heterogeneous. We also believe that a selection bias may have existed, in that patients who achieved better than a PR and were in durable remission went on to receive HDCT/autoPBSCT.
A variety of cytoreductive regimens have been used, however, it has been difficult to compare their efficacy because of inconsistencies in the definition of high-risk ESFT and limited number of cases. Burdach et al.8) reported a 6-year EFS of 45% for patients who received HDCT and total body irradiation (TBI) followed by stem cell rescue compared to 2% for the historical control group of patients who were treated with chemotherapy only. Non-TBI-containing regimens have also been reported to be superior to TBI-containing regimens and melphalan has long been the most commonly used2). From the EURO-EWING 99 trial, there was no difference of survival between cytoreductive regimens19). The 3-year EFS of the patients who received bulsulfan and melphalan was not statistically different from those received other HDCT regimens (32% vs. 20%)19). Traditionally, busulfan or melphalan have been used as a cytoreductive regimen, and recently, carboplatin, ifosfamide and thiotepa are used in the HDCT of ESFT9,10,15). And tandem transplantation appears to improve the outcome of patients with high-risk ESFT2,9,10). In this pilot study, we adopted carboplatin and thiotepa as alkylators and much-higher doses of etoposide. Patients were treated with this combination in the first course of HDCT, and in the second course, with classical regimen of melphalan and cyclophosphamide.
The 2-year EFS of 44.4% in our 9 patients is comparable to that of the Euro-American series2,5,7-9,12,19). The prognosis of ESFT in Asian, African, and Hispanic populations is reported to be poor20), and controversy exists as to whether similar treatment strategies produce similar results in low-incidence populations21,22). At present, limited data are available regarding the efficacy of HDCT in Asian patients with ESFT. Two Japanese studies evaluated the efficacy of HDCT in ESFT15,23). They reported that ESFT patients had a favorable outcome after a single course of HDCT (3-year EFS, 50%; 5-year relapse free survival, 81%). It is difficult to compare the efficacy of HDCT between case series, because of different eligibility criteria. We presume that the favorable outcomes of the 2 Japanese series could have partly been the result of including less severe cases, that is, those of an extremity tumor without metastasis. We have previously reported that survival of patients with localized ESFT treated at our institution was comparable to that reported for Euro-American cases17). Similarly, in this study we observed that the outcomes of Korean patients with high-risk ESFT treated with HDCT/autoPBSCT were similar to those of the Euro-American series.
The benefit of HDCT for ESFT is still inconclusive. HDCT/autoPBSCT improved the prognosis of recurrent or high-risk solid tumors in children11). The survival of patients with high-risk neuroblastoma has substantially increased with HDCT/autoPBSCT11,24), and for infants and young children with malignant brain tumors, HDCT maintained or improved survival while facilitating avoidance or deference of radiation therapy until 3 years of age25). Similarly, HDCT has been attempted to improve the survival of patients with high-risk ESFT. The first experience of HDCT for ESFT was in patients with metastatic disease, which resulted in improved survival from 0% (with conventional therapy) to 27%13). Since then, several groups have evaluated the efficacy of HDCT for ESFT, but have produced conflicting results. In each study, patients received HDCT based on alkylating agents6); however, the indication for the use of HDCT varied. In addition to metastatic4,8,13,14) or recurrent disease18,14), some studies included patients with centrally located tumors9,15,23) or poor responders to conventional chemotherapy10), and compared the results of HDCT to those of historical cases. To date, there have been no studies comparing HDCT and conventional chemotherapy in a prospective randomized setting.
We believe that survival of certain subgroups of patients may be improved with HDCT. The results of the EURO-EWING 99 trial provided valuable information19). The 3-year EFS of 127 patients with primary disseminated multifocal disease receiving busulfan and melphalan was similar to that in historical cases (32%). However, a scoring model based on clinical characteristics suggested that certain groups of patients may survive with intensive multimodal therapy. They reported that among the disseminated multifocal ESFT cases, young patients (<14 years), patients with small tumor volumes (<200 mL), and patients with a limited extent of metastasis had a better outcome after HDCT, with an EFS of up to 50%. Case no. 6 in our study, a 7.1-year-old girl who presented with multiple bone and bone marrow metastasis, was still disease free at 39 months after HDCT/autoPBSCT. She belongs to the low-risk score group defined in the EURO-EWING 99 report. This prognostic scoring model could be used in risk-adapted therapy for primary disseminated multifocal ESFT, for example, HDCT for low-risk cases and early experimental treatment for very high-risk cases.
Summarizing, we could not observe a clear benefit of HDCT/autoPBSCT for children and adolescents with high-risk ESFT. Disease status at the time of HDCT tended to influence the survival and durable remissions were hardly achieved after HDCT/autoPBSCT, without proper local tumor control. We believe that our study was inconclusive due to its retrospective nature and the small number of cases. Future studies should be conducted in a prospective and randomized design in order to define the role of HDCT as well as to identify a subgroup of patients who might benefit from this investigational treatment.
Notes
No potential conflict of interest relevant to this article was reported.