Toxic epidermal necrolysis induced by lamotrigine treatment in a child
Article information
Abstract
Toxic epidermal necrolysis is an unpredictable and severe adverse drug reaction. In toxic epidermal necrolysis, epidermal damage appears to result from keratinocyte apoptosis. This condition is triggered by many factors, principally drugs such as antiepileptic medications, antibiotics (particularly sulfonamide), nonsteroidal anti-inflammatory drugs, allopurinol, and nevirapine. Lamotrigine has been reported potentially cause serious cutaneous reactions, and concomitant use of valproic acid with lamotrigine significantly increases this risk. We describe a case of an 11-year-old girl with tic and major depressive disorders who developed toxic epidermal necrolysis after treatment with lamotrigine, and who was diagnosed both clinically and pathologically. Children are more susceptible to lamotrigine-induced rash than adults, and risk of serious rash can be lessened by strict adherence to dosing guidelines. Unfortunately, in our case, the patient was administered a higher dose than the required regimen. Therefore, clinicians should strictly adhere to the dose regimen when using lamotrigine, especially in children.
Introduction
Toxic epidermal necrolysis (TEN) is a life-threatening cutaneous reaction associated with 30% mortality1). Inappropriate immune activation is triggered in response to certain drugs or their metabolites. Numerous medications have been implicated as causes of TEN, the most frequently associated drugs include aromatic anticonvulsants, sulfonamide antibiotics, allopurinol, oxicam nonsteroidal anti-inflammatory drugs, and nevirapine2). Diagnosis relies mainly on clinical signs together with the histological analysis of a skin biopsy showing typical full-thickness epidermal necrolysis due to extensive keratinocyte apoptosis3).
Lamotrigine is a relatively new anticonvulsant. It is used for different types of epilepsy and also effective in treating and preventing bipolar depression. The risk of TEN with combination lamotrigine and valproate is greater than with monotherapy4). Children are more susceptible to lamotrigine-rash than are adults5).
TEN is rare disease with incidence of approximately 1.9 cases per million inhabitants annually based on all cases reported to the Food and Drug Administration (FDA) adverse effect reporting system databases in the USA6), and is also rare disease in Korea7). We report a case of TEN in a child that occurred after addition of lamotrigine to the valproic acid treatment, and who was diagnosed clinically and pathologically.
Case report
An 11-year-old girl was hospitalized for erythematous maculopapular rash with an itching sensation over the entire body with fever, conjunctival injection, eye wax, and oral ulceration. The patient had experience tic disorder since the age of 8 years, and had been diagnosed with major depressive disorder about 7 months previously. The patient was initially treated with fluoxetin, valproate, risperidone, and aripiprazole in psychiatric clinic. However, because of inadequate control, lamotrigine (25 mg/day) was added to the regimen about 3 weeks before hospitalization. After 2 weeks use of lamotrigine, the patient developed an erythematous rash, starting from the face and gradually spreading over the whole body.
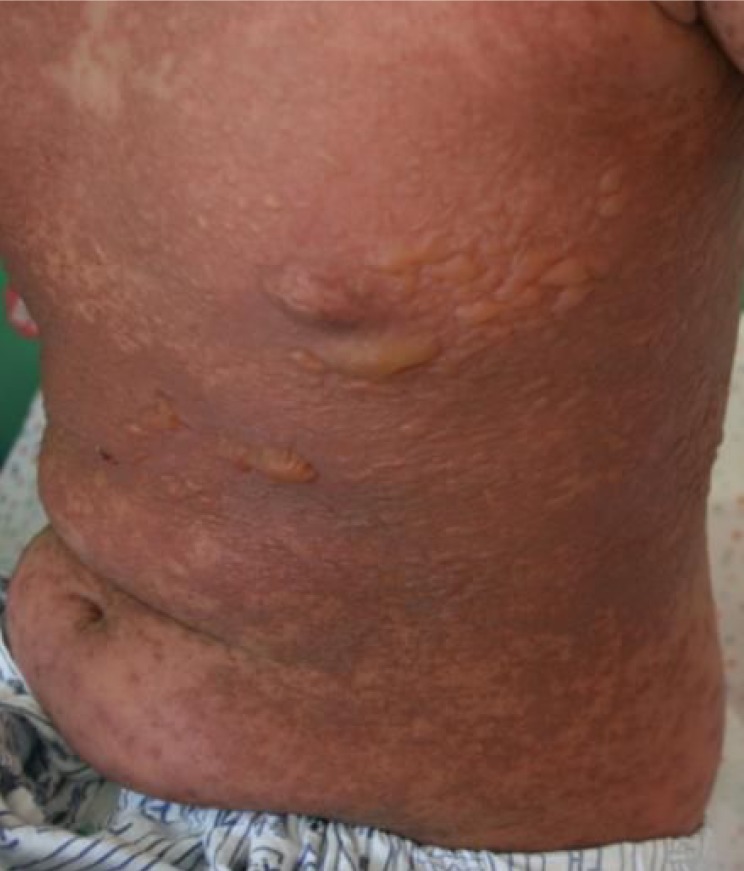
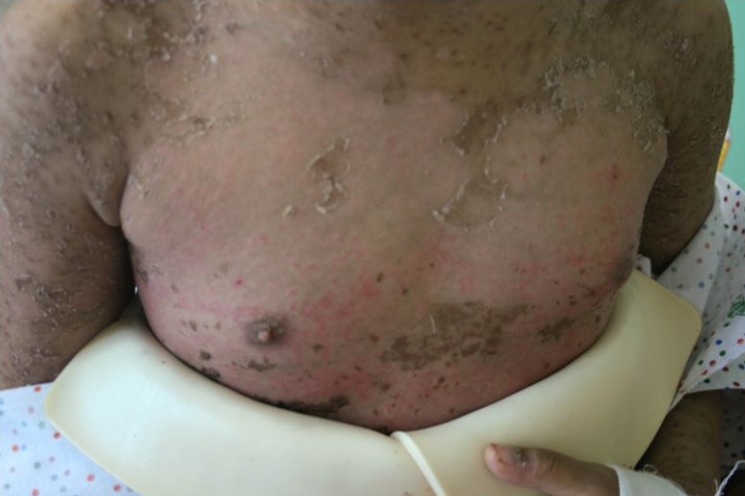
The patient transfered to Soonchunhyang University Hospital with a diffuse, confluent erythema, which covered over 90% of the total body surface area, conjunctival injection, eye wax, vesicle, and clot on lips and oral ulceration (Fig. 1). The patient also had a fever and skin tenderness, and could not eat properly because of oral pain. During the hospitalization, the skin lesion progressed to a purpuric rash with bullae (Fig. 2). Hemorrhagic crust on the lip was aggravated and Nikolsky's sign was positive. There was no history of food or drug allergy. Laboratory examination revealed normal complete blood count and serum biochemistry, normal erythrocyte sedimentation rate, and elevated C-reactive protein (7.56 mg/dL). Urinalysis showed pyuria (leukocyte 3+), but urine culture was negative. Chest x-ray was normal. Skin biopsy revealed necrotic keratinocyte in the epidermis with spongiosis and exocytosis, and lymphohistiocytic infiltration in the upper dermis, which suggested TEN (Fig. 3).

Diffuse and confluent erythema that covered ≥90% of the total body surface area of the patient on day 1 of hospitalization.

Histology of the skin biopsy specimen of the patient, showing necrosis of epidermal keratinocytes and lymphocytic infiltration in the upper dermis (H&E, ×200).
Consultations with a dermatologist, a plastic surgeon, a psychiatrist, an ophthalmologist and an otorhinolaryngologist were conducted. We stopped all the psychiatric medications and started intravenous (IV) antibiotics, suspecting secondary infection, intravenous immunoglobulin (IVIG;1.5 gm/kg/day for 3 days)8), and other conservative management including IV hydration, aseptic dressing, topical ointment, nasal irrigation using normal saline, oral humidification, and eye drops. After a week of hospitalization, the skin lesion showed desquamation and was improving (Fig. 4). The tic symptom recurred 2 weeks after we stopped the psychiatric medication, so we used aripiprazole. After 19 days of hospitalization, the lesion became much improved and the patient was discharged.
Discussion
TEN is an unpredictable and severe adverse drug reaction. Although several theories exist, the innate immune system is now favored as a significant contributor to the initiation and propagation of this devastating reaction9).
TEN is heralded by the abrupt onset of fever; systemic toxicity; a generalized, dusky, and erythematous rash; bullae; separation of large sheets of epidermis from the dermis; purulent conjunctivitis; and mucositis of the mouth and genital area2). In our case, the patient had oral, nasal mucosa and conjunctival involvements along with fever. Complete death of the epidermis leads to sloughing similar to that seen in large burns. TEN includes denudation of >30% of the total body surface area. Stevens-Johnson syndrome (SJS) affects <10% of the body surface, whereas involvement of 10%-30% of body surface area is called SJS/TEN overlap10). The estimated mortality rate for SJS is approximately 10%, while that for TEN is as high as 45%, with death mainly due to sepsis and hemodynamic failure11).
The prompt withdrawal of the suspected drug, fluid and electrolyte replacement, and topical wound care are the first line therapies. Administration of corticosteroids in TEN is controversial and even can be contraindicated12). The role of immunosuppressants, despite some success, is not well defined and is not considered as a standard. On the contrary, good results are reported in terms of decreasing mortality and morbidity or improving clinical conditions of the use of human IVIG13). Although some studies have shown no benefit from IVIG, the wealth of clinical experience supporting its therapeutic value cannot be dismissed. IVIG has minimal toxicities and, considering the gravity of the condition being dealt with, the risk/benefit ratio is quite favorable14). We started IV antibiotics and other conservative treatment at first, and on the day 2 of hospitalization commenced IVIG treatment because of aggravated skin lesion. We used IVIG for 3 days, and continued conservative treatment including aseptic dressing and topical ointment. The lesion showed improvement after a week of hospitalization.
Lamotrigine has been reported as having the potential to cause serious cutaneous reactions. Concomitant use of valproic acid with lamotrigine significantly increases the risk for development of adverse cutaneous reactions15). Valproic acid interacts with lamotrigine metabolism, leading to a reduced total clearance, and therefore to an increased elimination half-life of lamotrigine, resulting in higher serum concentrations16). Risk of serious rash may possibly be lessened by strict adherence to dosing guidelines. Children aged 2-12 years taking concomitant valproate and lamotrigine should be initiated at a dosage of 0.2 mg/kg once daily for the first two weeks, followed by 0.5 mg/kg once daily for the next 2 weeks, and increased thereafter by 0.5-1 mg/kg every other week to a maximum of 200 mg/day17). Unfortunately, in our case the patient initiated at the dosage of 25 mg (0.75 mg/kg) once daily. It would be important, if lamotrigine is added to valproic acid treatment, to initiate at a low dose and then increase slowly.
The use of lamotrigine in psychiatry has increased significantly after its approval by the FDA. Therefore not only pediatricians but also psychiatric clinicians should keep the strict dose regimen when they use lamotrigine especially in children.
Notes
No potential conflict of interest relevant to this article was reported.