Measurements of fractional exhaled nitric oxide in pediatric asthma
Article information
Abstract
Exhaled nitric oxide (NO) has been extensively investigated as a noninvasive marker of airway inflammation in asthma. The increased NO expression induced by inflammatory mediators in airways can be monitored easily in exhaled air from asthmatic children. Based on the relationship between the increased NO expression and eosinophilic airway inflammation, fractional exhaled nitric oxide (FeNO) measurements become an important adjunct for the evaluation of asthma. In addition, the availability of portable devices makes it possible to measure FeNO more easily and frequently in the routine pediatric practice. Despite various confounding factors affecting its levels, FeNO can be applicable in diagnosing asthma, monitoring treatment response, evaluating asthma control, and predicting asthma exacerbations. Thus, although pulmonary function tests are the standard tools for objective measurements of asthmatic control, FeNO can broaden the way of asthma monitoring and supplement standard clinical asthma care guidelines.
Introduction
Although effective asthma control is achieved in the majority of asthmatic patients, a significant numbers of patients still suffer from frequent asthma aggravations which are usually related to airway inflammation1,2). Moreover, patients whose asthma is considered under control still have evidences of airway inflammation3). These findings imply that the routine assessment of airway inflammation is required to ensure the more successful management of asthma. However, treatment of asthma based on sufficient evidences of airway inflammation is not usually instigated even in tertiary referral centers. Instead, the managements of asthmatic patients are usually based on symptoms, pulmonary function tests, assessment of bronchodilator response, and bronchial challenge tests4). However, symptoms or the results of basic pulmonary function tests may not reflect ongoing airway inflammation5,6). For these reasons, the development of monitoring modalities to assess airway inflammation is mandatory. In addition, measurements should be easy to perform, reproducible, and associated with a high degree of acceptance by patients.
Bronchoscopy with biopsy and induced sputum eosinophil count remain the best reliable tools for assessing airway inflammation. However, these monitoring methods are not always applicable as routine procedures because they are time consuming and accompanied by risks. More recently, fractional exhaled nitric oxide (FeNO) has been proposed as means of assessing airway inflammation. High FeNO values above certain cut-point may indicate active eosinophilic airway inflammation and the likelihood of deterioration in asthma control7). FeNO measurements have also shown potential utility in guiding anti-inflammatory therapy in asthmatic patients8-10). However, the clinical value of FeNO measurements may be questioned because FeNO levels are increased in asthma even in mild and asymptomatic conditions11,12). In addition, contribution of the possible sources of NO must be considered when exhaled NO is measured as a marker of airway inflammation. This review focuses on the basic features of exhaled NO and the role of FeNO measurements in pediatric asthma.
Basic features of NO production
Nitric oxide (NO) had been considered to be an air pollutant without any specific biological roles. However, NO is involved in a broad range of physiologic and pathologic processes. NO is produced by a variety of cells including airway epithelial cells, vascular endothelial cells, and trafficking inflammatory cells. NO is usually synthesized by NO synthase (NOS) which catalyzes the oxidation of L-arginine to NO and L-citrulline13) (Fig. 1). Neural NOS (NOS1), inducible NOS (iNOS, NOS2), and endothelial NOS (NOS3) are three different NOS isoenzymes14). Constitutive NOS (cNOS) isoenzymes include NOS1 and NOS3, both of which are activated by calcium ions to produce small amounts of NO, which is presumed to play a local regulatory role, such as neurotransmission (NOS1) and regulation of local blood flow (NOS3)15).
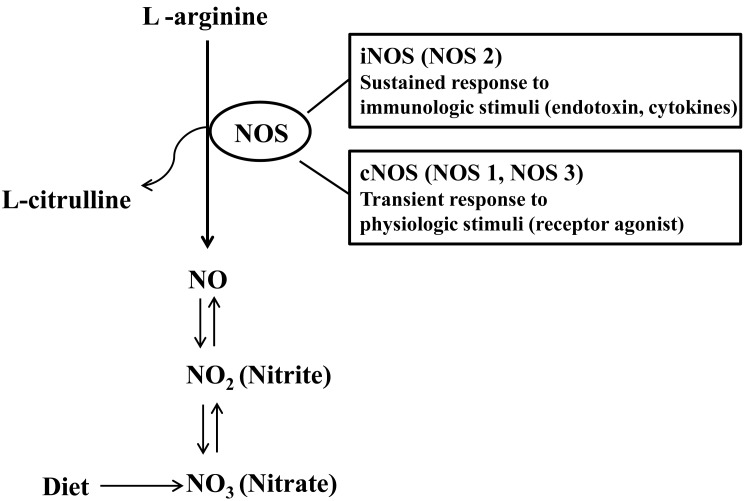
Nitric oxide synthetase (NOS)-dependent and -independent formation of nitric oxide (NO). iNOS, inducible NOS; cNOS, constitutive NOS.
In contrast to cNOS inducing NO production at picomolar concentrations for the transient period, iNOS is able to produce NO in nanomolar concentrations on a continuous basis16), reflecting that iNOS is the most important isoenzyme in determining NO output. In addition, iNOS is not constitutively expressed but induced by inflammatory or infectious stimuli and produces NO independent of calcium ion influx. The expression of iNOS is higher in the upper airways than in the lower ones and regulated at the transcriptional level17). Expression of iNOS in respiratory epithelium has been shown to be upregulated by Th2 cytokines via the STAT-6 pathway18). Asthmatic patients exhibit higher levels of iNOS mRNA expression than healthy controls. The expression of iNOS is decreased after the use of corticosteroid in patients with asthma. Close relationship between FeNO and epithelial iNOS mRNA expression supports that the increased expression of epithelial iNOS is responsible for high FeNO19).
Factors affecting FeNO levels
High FeNO is observed primarily in atopic asthma, which presents a contrast to normal or near-normal FeNO in nonatopic asthma20). In addition, FeNO has been shown to be positively correlated with the level of IgE sensitization21) and elevated after controlled or natural exposure to allergens22). In this regard, inhaled allergens are probably the most important environmental agents enhancing FeNO levels particularly in atopic subjects. Previous studies have shown mixed results concerning interrelationship among asthma, atopy and FeNO. Some studies reported high FeNO mainly confined to atopic children independent of asthma status23,24), while others have found higher FeNO levels in atopic subjects with concomitant asthma than those with atopy alone20,25).
NO can be formed from nonenzymatic NOS-independent sources via reduction of NO metabolites26). Consumption of green vegetables with high amounts of nitrate can result in higher levels of NO in exhaled breath. The increase of exhaled NO seems to be proportional to the amount of ingested nitrate and last up to 15 hours after intake27). Therefore, it is required to instruct patients to avoid large amount of nitrate-rich food on the day of measurement and encourage a mouthwash as this minimizes the effect of nitrate on FeNO.
Although the results about the effect of physical exercise on FeNO are conflicting, it is better to refrain from physical exercise 1 hour before FeNO measurements to minimize the effect of physical exercise28). Cigarette smoking is associated with the reduced levels of FeNO, although FeNO is still high in smokers with asthma26). Air pollution such as elevated levels of particulate matter or ozone is associated with the increase of FeNO both in healthy subjects and asthmatics29,30).
Rhinovirus (RV) infection has been known be a major cause of asthma exacerbation and appears to be associated with systemic eosinophilic activation leading to sputum eosinophilia. RV can induce iNOS expression in the airway epithelium resulting in enhanced NO production31). NO response to RV infection is probably beneficial because NO suppress viral replication. Interestingly, asthmatic children with RV infection had lower FeNO than those without RV infection when their asthma was exacerbated32), suggesting that RV infection is unlikely to be involved in Th2-driven iNOS expression.
Corticosteroids can reduce FeNO in asthmatic patients probably by inhibiting the activity of transcriptional factor GATA-3 which regulates interleukin (IL) 4/IL-13 expression in immune cells including Th2 lymphocytes33). However, corticosteroids are not able to inhibit epithelial STAT-6 expression which is involved in iNOS expression34). Because FeNO appears to be a marker for allergen-induced local airway inflammation, it can monitor the efficacy of different inhaled corticosteroids (ICS) preparations in the treatment of asthma. However, FeNO measurements may be less useful in monitoring asthmatic patients who are frequently exposure to specific allergens because FeNO remains high despite continuous use of ICS in these patients. Leukotriene receptor antagonists can reduce FeNO in asthmatic patients although this effect is less profound than corticosteroids35). In contrast, theophylline and chromorines are not able to affect the level of FeNO36,37).
The levels of FeNO can be changed by bronchoconstriction and bronchodilation, implying a dependence of FeNO on airway caliber which is related to airway mucosal surface area for NO diffusion. Thus, continuous use of long-acting β2-agonist in asthmatic patients can result in increase of FeNO38).
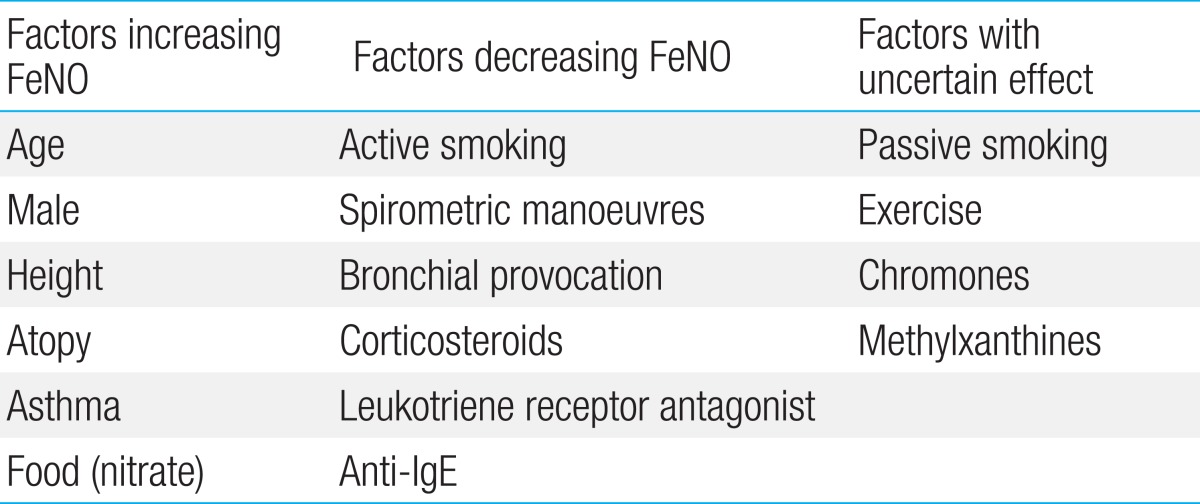
Despite inconsistent results concerning a sex difference in FeNO levels, there appears to be an association between female sex and lower levels of FeNO26). This might be explained by lower endogenous NO production and smaller surface area of the conductive airways in relation to body size in females. Height has been reported to be one of the most independent confounder for FeNO. In a previous study performed in children, an increase from 120 to 180 cm resulted in a doubling of exhaled NO levels, reflecting the greater airway mucosal surface area for NO diffusion as children grow39). By the same token, increasing age is associated with an increase of exhaled NO in children. However, the influence of weight or body mass index on FeNO remains unclear because of mixed results in previous studies. Factors and their effects on FeNO are summarized in Table 1.
Methods of FeNO measurements
Exhaled NO is usually measured by chemiluminescence or electrochemical sensing. The chemiluminescence method was first used for detection of exhaled NO in the breath of humans and became the gold standard for FeNO measurements. However, the chemiluminescence-based NO analyzer is expensive and not portable. On the other hand, NO analyzers with electrochemical sensors are hand held devices and relatively cheaper than those based on chemiluminescence. Because there is a good agreement in FeNO levels between both devices, the use of hand held devices becomes increasing40).
After inhalation to total lung capacity, patients must exhale immediately into the NO analyzer until a steady plateau is reached. Because FeNO levels are flow dependent41), exhalation should be stable at a targeted flow rate of 50 mL/sec and last for 6-10 seconds. Higher exhalation flow rate as well as shorter exhalation time results in FeNO lower than true values. NO scrubber is usually used to ensure that the patients inhale NO-free air. According to the 2005 American Thoracic Society/European Respiratory Society guidelines28), FeNO should be measured twice and a third measurement is required if there is a more than 10% difference between first two measurements. In addition, guidelines also recommend avoiding spirometric maneuver before FeNO measurements because this maneuver can lower FeNO. Thus, spirometry should be performed after FeNO measurements. Nasal clip should not be used as it can affect closure of the soft palate, leading to contamination with NO derived from the nasal cavity. In children younger than 5 years, single-breath online measurement is not well standardized since children cannot adequately cooperate. In very young children who cannot maintain a constant flow, FeNO levels are measured during spontaneous breathing, with the exhalation flow adjusted to 50 mL/sec by changing the exhalation resistance42).
Clinical application of FeNO in pediatric asthma
Based on its correlation with bronchial hyperresponsiveness43), FeNO may be considered as a screening tool for asthma. Actually, sensitivity and specificity of FeNO measurements have been reported to be acceptable for the diagnosis of asthma in previous clinic-based studies44,45). In particular, FeNO provides significantly higher diagnostic accuracy than lung function tests in discriminating asthmatics from healthy subjects44,46). The power of FeNO in diagnosing asthma can be increased further by the combination of airway hyperresponsiveness and elevated FeNO47). However, it is important to remember that low FeNO values do not exclude the diagnosis of asthma because FeNO levels are normal, especially in nonatopic subjects7). In addition, because severity or phenotype of airway inflammation may be different among patients with atopic asthma, FeNO levels may be different among these patients20).
Prior publications have reported reference values for FeNO in healthy children and adolescents48-50). However, because of multiple confounding factors influencing FeNO, normal predictive values for FeNO might be relatively poor and have large confidence intervals. According to the 2011 American Thoracic Society guideline for interpretation of FeNO for clinical applications51), asthma with eosinophilic inflammation is less likely in children who have FeNO below 20 ppb. In contrast, FeNO levels higher than 35 ppb in children strongly suggest airway eosinophilia. Thus, discriminating asthma from nonasthmatic conditions in children with intermediate FeNO levels of 20-35 ppb warrants cautious interpretations. For these children with symptoms suggesting asthma, the change of FeNO from the personal best FeNO value may be helpful in interpreting the level of airway inflammation because the intrasubject reproducibility of FeNO levels appears to be good52). Based on currently available data, algorithms for interpretation and clinical application of FeNO results can be developed for diagnostic use and ongoing asthma management (Fig. 2).
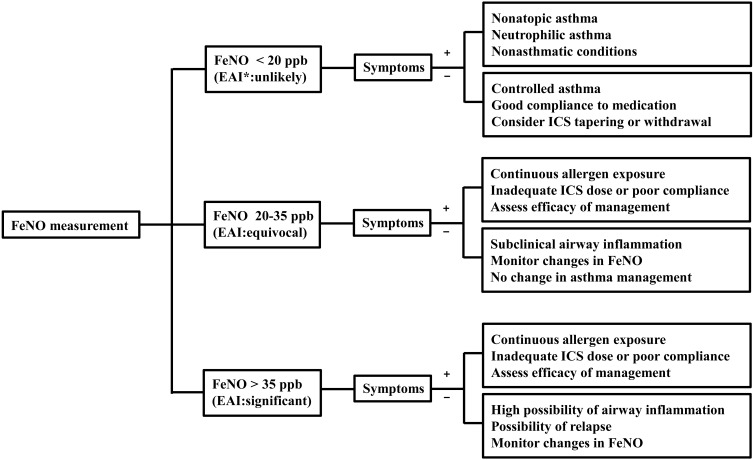
Clinical application and interpretation of FeNO levels in pediatric asthma. FeNO, fractional exhaled nitric oxide; EAI, eosinophilic airway inflammation; ICS, inhaled corticosteroids.
FeNO correlates with airway eosinophilia in induced sputum, biopsy material, and bronchoalveolar lavage fluid53,54). Therefore, FeNO measurements may be a useful monitoring tool for eosinophilic airway inflammation, without the practical difficulties associated with bronchial biopsy or sputum induction. Furthermore, high FeNO at the time of loss of asthma control55,56) and decrease of FeNO after treatment with corticosteroids57,58) imply that FeNO is useful not only in predicting asthma exacerbation but in monitoring the response to treatment. The changes in FeNO levels during use of inhaled and oral corticosteroids are rapid and reproducible when repeated57). In this regard, FeNO measurements may be helpful in adjusting ICS dose in asthmatic individuals. However, because previous clinical trials reported only equivocal benefits of adding FeNO measurements to usual clinical guidelines for asthma management8-10,59), it has not been determined that FeNO-based management is beneficial in routine clinical practice. In addition, despite the increase of FeNO prior to asthma exacerbation, evidences are not sufficient enough to suggest that FeNO measurements are able to role as a predictor of asthma exacerbation60,61). Nevertheless, changes in FeNO levels from baseline and individually tailored FeNO values may be both good markers for the upcoming loss of asthma control62).
Although asthma aggravation is accompanied by increased symptoms, impaired lung function, and high FeNO levels, there may be discordance among these asthma outcomes. In particular, monitoring of asthma control based on symptoms and normal forced expiratory volume in 1 second might result in under-treatment because persistent airway inflammation does not necessarily manifest itself as symptoms or lung malfunction6,63). High FeNO levels in the absence of symptoms or lung function abnormalities raise the possibility of active eosinophilic airway inflammation and the likelihood of deterioration in asthma control. Thus, in cases of silent airway inflammation, monitoring with FeNO measurements might potentially allow for better targeting and monitoring of anti-inflammatory treatment64). However, further studies are required to elucidate whether subclinical airway inflammation identified by FeNO measurements needs to be treated.
Conclusions
FeNO is a noninvasive surrogate marker of eosinophilic airway inflammation and responds rapidly to changes in the inflammatory state of the airways. Therefore, combined with other asthma outcomes, FeNO can provide additional information about the ongoing status of asthma. However, although FeNO measurements offer a step forward in the assessment of asthma, the interpretation of FeNO values in patients with asthma needs to be further clarified. Nevertheless, FeNO has a potential to serve as a tool for diagnosing and monitoring pediatric asthma.
Acknowledgments
This work was supported by the research grant of the Chungbuk National University in 2012.
Notes
No potential conflict of interest relevant to this article was reported.