Primary repair of symptomatic neonates with tetralogy of Fallot with or without pulmonary atresia
Article information
Abstract
Recently, surgical outcomes of repair of tetralogy of Fallot (TOF) have improved. For patients with TOF older than 3 months, primary repair has been advocated regardless of symptoms. However, a surgical approach to symptomatic TOF in neonates or very young infants remains elusive. Traditionally, there have been two surgical options for these patients: primary repair versus an initial aortopulmonary shunt followed by repair. Early primary repair provides several advantages, including avoidance of shunt-related complications, early relief of hypoxia, promotion of normal lung development, avoidance of ventricular hypertrophy and fibrosis, and psychological comfort to the family. Because of advances in cardiopulmonary bypass techniques and accumulated experience in neonatal cardiac surgery, primary repair in neonates with TOF has been performed with excellent early outcomes (early mortality<5%), which may be superior to the outcomes of aortopulmonary shunting. A remaining question regarding surgical options is whether shunts can preserve the pulmonary valve annulus for TOF neonates with pulmonary stenosis. Symptomatic neonates and older infants have different anatomies of right ventricular outflow tract (RVOT) obstructions, which in neonates are nearly always caused by a hypoplastic pulmonary valve annulus instead of infundibular obstruction. Therefore, a shunt is less likely to preserve the pulmonary valve annulus than is primary repair. Primary repair of TOF can be performed safely in most symptomatic neonates. Patients who have had primary repair should be closely followed up to evaluate the RVOT pathology and right ventricular function.
Introduction
Tetralogy of Fallot (TOF) is the most common form of cyanotic congenital heart disease. It is characterized by four distinct anatomic features: (1) pulmonary outflow tract obstruction (stenosis or atresia), (2) ventricular septal defect (VSD), (3) overriding aortic root, and (4) right ventricular hypertrophy1-3). Since Fallot's description in 1888, treatments have included the so-called blue baby operation or Blalock-Taussig shunt, which was the initial surgical treatment for TOF, first performed by Blalock in 19454). This was followed by the first successful intracardiac repair using human cross-circulation, performed by Lillehei in 19545) and supplemented with a pump oxygenator by Kirklin et al.6) in 1955. For at least 2 decades, initial palliation followed by repair later in childhood was the most prevalent strategy. By the early 1980s, primary repair in early infancy had been advocated by Castaneda et al.7), Barratt-Boyes and Neutze8), and others. This approach gained wide acceptance and many surgeons adopted a therapeutic strategy based on early primary repair in the absence of specific anatomic contraindications or comorbidities such as major noncardiac anomalies. The concept of early primary repair was extended to symptomatic neonates and low operative mortality was achieved9-13).
However, the management of symptomatic infants with TOF requiring surgical intervention in the first month of life remains controversial14). In this article, I summarize my personal experience and review the literature concerning current practice of early primary repair in TOF neonates with pulmonary stenosis or atresia.
Considerations of the anatomy and pathophysiology of TOF in young infants requiring early intervention
TOF may be defined on the basis of antero-cephalad deviation of the outlet septum with associated malformation of the septoparietal trabeculation or, alternatively, may result from underdevelopment of the subpulmonary infundibulum2,15). A single abnormality or pathologic process during embryogenesis produces right ventricular outflow tract (RVOT) obstruction as well as malalignment-type VSD and aortic override. Right ventricular hypertrophy is the hemodynamic consequence of the above anatomical lesions16).
TOF displays a wide spectrum of disease, ranging from mild to severe RVOT obstruction. TOF with pulmonary atresia represents an extreme obstruction may occur at 1 or more of the following structures: (1) infundibulum, (2) pulmonary valve, (3) main pulmonary artery, and/or (4) branch pulmonary arteries3).
In patients with TOF with pulmonary stenosis, the initial manifestation of symptoms depends on the degree of RVOT obstruction17). Most commonly, cyanosis is mild at birth and gradually progresses with age as the stenosis increases in concert with increasing infundibular hypertrophy. Cyanosis tends to become significant within the first 6 to 12 months of life. In such situations, the obstruction is entirely or predominantly at the infundibular level. The pulmonary valve annulus and the branch pulmonary arteries usually are of good size. However, a smaller percentage of patients have marked cyanosis at or soon after birth. In this group, the RVOT obstruction is nearly always caused by a hypoplastic pulmonary valve annulus12,17,18). Cyanosis is constant in these patients because of the fixed nature of the obstruction to pulmonary blood flow. Castaneda et al.7) described the anatomy of RVOT in young infants with TOF presenting with cyanosis. Although the infundibulum was stenotic, the area of the greatest obstruction to pulmonary blood flow in the majority of infants was at the pulmonary valve annulus, in which the pulmonary valve was bicuspid or dysplastic with fusion of commissures.
Pulmonary atresia is present in approximately 7% of patients with TOF19). TOF with pulmonary atresia exhibits considerable morphologic variability, particularly with respect to the source of pulmonary arterial flow20). When patients with pulmonary atresia have no major collateral arteries, a patent ductus arteriosis is the only source of pulmonary blood flow. In such cases, there may be varying degrees of hypoplasia of the pulmonary arteries, but it is most often the case that all or nearly all lung segments are supplied by branches arborizing from the right and left pulmonary arteries; that is, pulmonary arborization is much more preditable21).
Surgical options in symptomatic TOF neonates
Symptomatic TOF may be prostaglandin-dependent or be characterized by either hypoxic episodes or severe hypoxemia with resting systemic oxygen saturation of less than 75%. Neonates born with TOF with pulmonary stenosis develop early symptom uncommonly, but a small percentage of patients do have marked cyanosis at or soon after birth, as previously mentioned. TOF neonates with pulmonary atresia may be symptomatic or asymptomatic, but inevitably have duct-dependent pulmonary circulation. For such patients, surgical options include early primary repair or palliation with an aortopulmonary shunt.
Recently, Al Habib et al.22) analyzed contemporary patterns of management of TOF with pulmonary stenosis by using The Society of Thoracic Surgeons (STS) Database (3,059 operations in 2002-2007). This study showed that primary repair in the first year of life is the most prevalent strategy. However, for the neonatal age group, primary procedures were about equally divided into primary repair and palliation (154 cases vs. 178 cases among a total of 332 procedures, respectively). This demonstrates that determining an optimal strategy for neonates with symptomatic TOF remains an unresolved issue up to the present.
Aortopulmonary shunts in neonates with symptomatic TOF
For neonates and young infants with symptomatic TOF, many surgeons perform palliation with an aortopulmonary shunt. Those who prefer this option are likely to agree with the use of primary repair in TOF at 3 months of age or older. Recently, Kanter et al.14) reported a study comparing the surgical options of primary repair and shunting in symptomatic TOF neonates. They concluded that these options resulted in equivalent mortality and outcomes, although shunted patients had fewer transannular patch repairs despite having more emergent initial operations.
At this point, two issues should be addressed regarding shunting in neonates with TOF: (1) the safety of shunting in these patients and (2) whether shunting additionally preserves the pulmonary annulus.
1. Safety issues of shunting
In infants with TOF, palliative shunting has been performed safely and with excellent outcomes23,24). Even in young infants, this option still provides good outcomes similar to those of primary repair14,22). Recently, Kanter et al.14) reported excellent early outcomes including shorter intensive care unit and hospital stays for the first operation and early mortality of 5.9%. According to the STS database, discharge mortality from TOF repair in neonates was 11 of 178 (6.2%) for palliation and 12 of 154 (7.8%) for primary repair22). However, outcomes of shunting in neonates have been reported inconsistently and with fluctuations. In a multicenter study of the effect of aspirin on shunting, clinical outcomes of palliative shunts in infants were poor even in the current era25). This study showed that event rates of shunt thrombosis and death were 5% and 15%, respectively, for TOF patients and 16% and 21%, respectively, for patients with pulmonary atresia. Recently, Guzzetta et al.26) reported outcomes of in-hospital shunt occlusion in 207 young infants undergoing only a modified Blalock-Taussig shunt, in which in-hospital shunt occlusion occurred in 14 patients (6.8%). Patients who had shunt occlusion had a harder postoperative course and a higher rate of in-hospital mortality (6.2% vs. 21.4%). They concluded that the risk factors of in-hospital shunt occlusion were cardiac diagnosis (pulmonary atresia) and the size of the pulmonary arteries.
With regard to the safety evaluation, interim mortality should be addressed in infants undergoing shunting13,27,28). Interim mortality occurs during the palliated state before repair and may account for additional attrition, which may or may not be shunt-related29).
2. Issues of preservation of pulmonary valve annulus in neonatal TOF repair
For TOF neonates with pulmonary stenosis, the influence of shunting on the growth of the pulmonary annulus should be evaluated. Pulmonary regurgitation after TOF repair results in various adverse long-term outcomes30). Therefore, every effort should be made to preserve total pulmonary valve function as well as the pulmonary annulus. Recently, Kanter et al.14) suggested that shunted patients have a greater likelihood of avoiding a transannular patch at the time of repair. Sousa Uva et al.31) reported that initial palliation promoted the growth of pulmonary annulus and that transannular patching was less prevalent for patients who underwent initial palliation (13% vs. 56%, P=0.03). One year later, however, in a report on the same issue, the same authors revised this conclusion, stating that the observation of increased size of the pulmonary annulus after shunting could be due to chance only and that initial palliation did not allow for a reduction in incidence of transannular patching32). The incidence of transannular patching at the time of primary repair in neonates ranges from 84% to 100%10,12,14,33). Symptomatic neonates usually receive a transannular patch because of their morphology, not because of their age. The need for a transannular patch reflects the severity of the RVOT obstruction at the annular level7,10,12,18). Parry et al.18) reported outcomes of elective primary repair of acyanotic TOF in early infancy, in which only 28% of patients required transannular patching. Follow-up echocardiography suggested a trend towards 'catch-up' growth of the annulus. By contrast, in a disease entity distinct from TOF, Emani et al.34) showed progressive decrease in the pulmonary valve annular size in cases of delayed anatomic repair beyond the neonatal period. They suggested that antegrade blood flow may provide the stimulus for growth of ventricular outflow tract structures, while palliative maneuvers such as aortopulmonary shunts may lead to a reduction in blood flow through the outlet area.
Primary repair in symptomatic TOF neonates
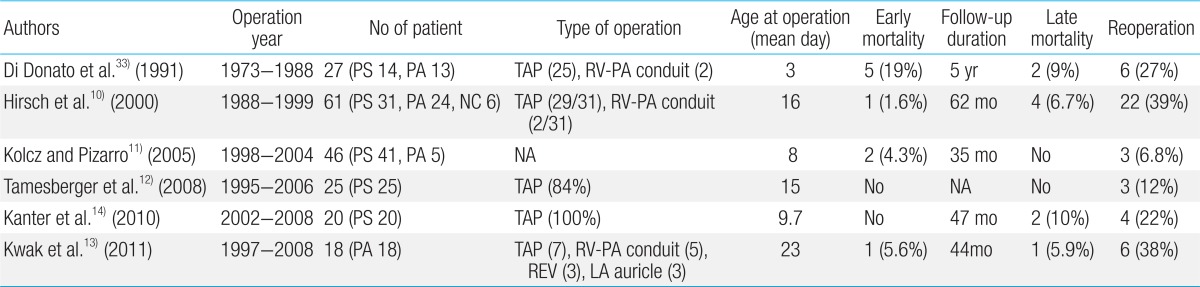
An initial study by Kirklin et al.35) suggested that primary repair of TOF at less than 3 months of age was associated with a high mortality. Over the past several years, however, increasing success with primary repair of TOF in younger infants has been demonstrated by many centers9,18,32,36). In recent reports, early primary repair in symptomatic neonates with TOF has been performed with excellent early outcomes (Table 1).
Early primary repair provides multiple advantages, including avoidance of shunt-related complications, early relief of hypoxia, promotion of normal lung development, avoidance of ventricular hypertrophy and fibrosis, and psychological comfort to the young family37,38).
In the past, indications for delayed repair included anomalous coronary artery crossing of the RVOT, hypoplastic or discontinuous pulmonary arteries, and multiple VSDs39). However, in the current ear, there are essentially no contraindications to early primary repair38,39).
For symptomatic neonates with TOF, Kanter et al.14) suggested that those who were younger and required emergency operation should be shunted and those with favorable anatomy and good-sized branch pulmonary arteries should have primary repair. Shunting in smaller neonates requiring emergency operation is not absolutely safe and most likely is associated with considerable mortality and morbidity. Recent accumulation of experience in neonatal cardiac surgery and advances in cardiopulmonary bypass techniques may provide more stable outcomes after primary repair in such situations.
The issue of the size of pulmonary arteries should be simplified to the identification of the presence of major aortopulmonary collateral arteries. If there are no major collateral arteries present, the pulmonary arteries are adequate for primary repair of TOF38). Jonas38) emphasized that since the pulmonary arteries are underfilled and underpressurized preoperatively, the potential size of the pulmonary arteries is unknown no matter what imaging technique is used. Van Arsdell and Yun40) pointed out that problems with truly small pulmonary arteries are infrequent (1%-2%) and can be managed with a fenestrated VSD.
Surgical techniques for primary repair in neonatal TOF have been progressively changed over the last several decades. In the early years, neonatal TOF repair was performed using cardiopulmonary bypass with a period of deep hypothermic circulatory arrest7,10,11,33). Recently, however, many surgeons have tried to avoid deep hypothermic circulatory arrest and instead perform primary repair under continuous moderate hypothermic cardiopulmonary bypass and cardiac arrest12-14). The VSD is closed through a transatrial and/or transventricular approach. RVOT reconstruction is performed by using various techniques, including transannular or nontransannular patching in pulmonary stenosis and transjunctional patching, interposition of a conduit from the right ventricle to the pulmonary arteries, and other techniques in pulmonary atresia. Farouk et al.20) described these surgical techniques in patients with TOF with pulmonary atresia as well as the feasibility of a transatrial approach for VSD closure in all patients.
Personal experience of primary repair of TOF with or without pulmonary atresia
My personal strategy for surgical intervention in neonatal TOF is to implement early primary repair if possible. I have reviewed my clinical experience of surgical treatment in symptomatic neonates with TOF.
1. Patient population
Between May 2004 and December 2012, 27 consecutive neonates with TOF underwent a surgical intervention. Their cardiac diagnoses were TOF with pulmonary stenosis (n=6) or atresia (n=21) with no major aortopulmonary collateral vessels.
All patients had symptoms before surgery. Twenty-six patients were receiving an infusion of prostaglandin. The remaining patient with pulmonary stenosis suffered an anoxic spell and was treated with propranolol and mechanical ventilator care. Ten patients needed mechanical ventilator care temporarily or until repair.
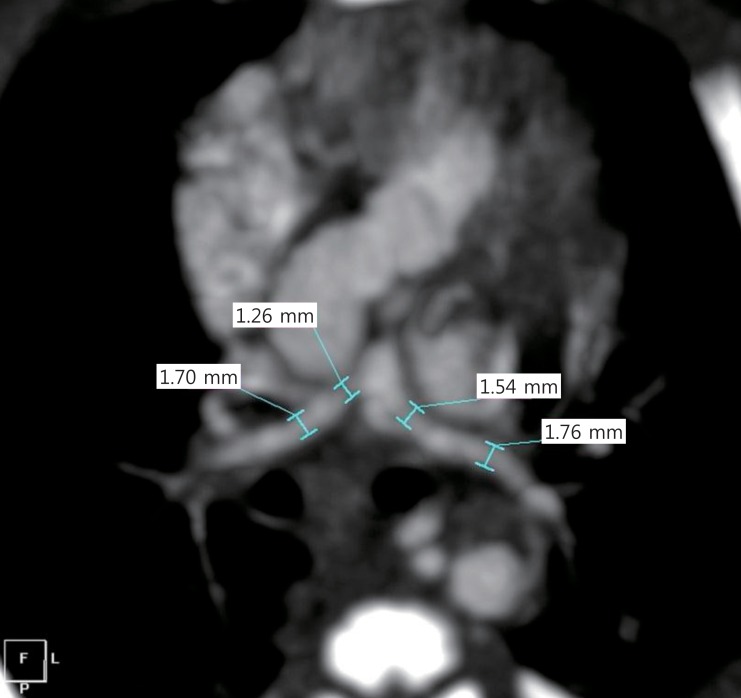
Intracardiac anatomy and the RVOT were assessed preoperatively by using transthoracic echocardiography. The morphology and size of the pulmonary arteries and the presence of major collateral arteries were assessed by performing chest computed tomography (CT) scan. Preoperative chest CT scan images were available to measure the size of the branch pulmonary arteries in 24 patients. McGoon ratio41) and Nakata index42) had median values of 1.1 (range, 0.6 to 1.5) and 121 mm2/m2 (range, 27 to 189 mm2/m2), respectively (Fig. 1). The single patient who had undergone shunting for small pulmonary arteries had a McGoon ratio of 0.6 and a Nakata index of 27 mm2/m2 (Fig. 1, black triangle*).

Preoperative size of the pulmonary arteries. Black triangle*, palliative case due to hypoplastic pulmonary arteries; black triangle**, palliative case due to apparent left ventricle hypoplasia.
Twenty-five neonates (93%) underwent primary repair. The median age at repair was 16 days (range, 12 to 29 days) and median body weight was 3.2 kg (range, 2.2 to 4.2 kg). Two patients (7%) had aortopulmonary shunts. One of these had a seemingly small LV size; the other had diffuse hypoplastic branch pulmonary arteries despite having no major aortopulmonary collateral vessels (Fig. 2).
2. Operative techniques
The early primary repair included VSD closure (a transatrial approach in 20 and a transventricular approach in 5), resection of the hypertrophied right ventricular muscle and RVOT reconstruction with various techniques. These included transannular or transjunctional RVOT widening with an autologous pericardial patch (n=12), interposition of a conduit made with an autologous pericardial roll between the right ventricle and the pulmonary arteries (n=8, 7 to 10 mm in diameter), RVOT reconstruction using the left atrial auricle as a flap (n=3), and REV (reparation a l'etage ventricularie)-type reconstruction (native tissue to tissue anastomosis between the posterior wall of the main pulmonary artery and right ventricle, n=2). No materials for preventing pulmonary regurgitation such as artificial monocuspid or bicuspid pulmonary valves were used in any patients. Fenestration of VSD was not necessary in any patient.
3. Cardiopulmonary bypass data
All primary repairs were performed after standard median sternotomy by using full-flow cardiopulmonary bypass with moderate systemic hypothermia. Antegrade crystalloid cardioplegia was used for myocardial protection. Median cardiopulmonary bypass and aortic cross clamp times were 153 minutes (range, 95 to 257 minutes) and 75 (range 50 to 117 minutes) minutes, respectively. Total circulatory arrest was not necessary in any patient.
4. Early results and postoperative course
There were no hospital deaths. Delayed sternal closure was necessary in 6 patients and postoperative complications requiring an interventional operation occurred in 5 patients. These complications comprised left-side diaphragm palsy (n=3), postoperative bleeding (n=1) and wound infection (n=1). No patient developed junctional ectopic tachycardia or complete atrioventricular heart block.
5. Late results
Follow-up was performed at a median interval of 56 months (range, 2 to 105 months). There were 2 late deaths. Actuarial survival rates at 6 months, 1 year, and 5 years were 96%, 92%, and 92%, respectively. Among 23 late survivors, 15 patients (65%) have not needed further surgical intervention after the primary repair. Eight patients have undergone surgical procedures of RVOT at a median interval of 14 months after total repair (range, 6.8 to 51 months) and 3 patients have needed second re-do operations for RVOT stenosis. The first catheter intervention for RVOT obstruction was performed after primary repair in 14 patients (61%) at a median interval of 7.4 months (range, 3 to 19 months). This was performed for left pulmonary artery stenosis in 6 patients, for right pulmonary artery stenosis in 2 patients, for stenosis of both branch pulmonary arteries in 5 patients, and for pulmonary trunk stenosis in 1 patient. Eight patients needed further catheter interventions such as reballooning or placement of a stent. Reoperation-free survival rates at 6 months, 1 year, and 5 years were 100%, 81%, and 60%, respectively, and catheter plus surgery intervention-free survival rates at 6 months, 1 year, and 5 years were 79%, 50%, and 33%, respectively. At the most recent follow-up, echocardiography of right and left ventricular systolic function was normal in all patients.
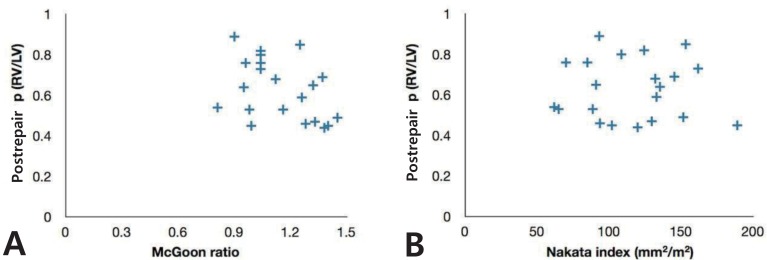
In 23 late survivors, the postrepair RV/LV pressure ratio was 0.61±0.15 (range, 0.37 to 0.89), unrelated to preoperative size of the pulmonary arteries (Fig. 3). However, for patients with ratios of 0.6 or more, catheter plus surgical interventions were needed significantly more frequently (10/11, 91% vs. 5/12, 42%; P=0.027 by Fisher exact test).
Conclusions
The optimal surgical strategy for symptomatic TOF neonates, whether primary repair or shunting followed by repair, remains elusive. The shunt operation is no longer considered a safe option in neonates, and additionally is less likely to save the pulmonary valve annulus. Primary repair of TOF can be performed safely in most symptomatic neonates. Patients who have had early primary repair should be closely followed up to evaluate the RVOT pathology and right ventricular function.
Notes
No potential conflict of interest relevant to this article was reported.