Chronic Epstein-Barr virus infection causing both benign and malignant lymphoproliferative disorders
Article information
Abstract
The Epstein-Barr virus (EBV) is oncogenic and can transform B cells from a benign to a malignant phenotype. EBV infection is also associated with lymphoid interstitial pneumonia (LIP). Here, we report the case of a 14-year-old boy who was diagnosed with a latent EBV infection and underlying LIP, without any associated immunodeficiency. He had been EBV-seropositive for 8 years. The first clinical presentations were chronic respiratory symptoms and recurrent pneumonia. The symptoms worsened in the following 2 years. The results of in situ hybridization were positive for EBV, which led to a diagnosis of LIP. The diagnosis was confirmed by the results of a thoracoscopic lung biopsy. The EBV titer of the bronchoalveolar lavage specimens obtained after acyclovir treatment was found to be fluctuating. The patient had latent EBV infection for 8 years, until presented at the hospital with intermittent abdominal pain and distension. Physical examination and pelvic computed tomography revealed a large mesenteric mass. A biopsy of the excised mass led to a diagnosis of Burkitt's lymphoma (BL). The patient received combination chemotherapy for 4 months, consisting of vincristine, methotrexate, cyclophosphamide, doxorubicin, and prednisolone. He is now tumor-free, with the LIP under control, and is being followed-up at the outpatient clinic. This is the first report of a Korean case of chronic latent EBV infection that developed into LIP and BL in a nonimmunocompromised child.
Introduction
Epstein-Barr virus (EBV) was the first human virus to be associated with malignancy. The virus is ubiquitous and often acquired by oral contact early in life. In developing countries, EBV infection is most common in populations of low socioeconomic status. EBV seroprevalence tends to increase gradually with age, showing two seroconversion peaks at 2-4 and 14-18 years of age1). The mean level of seroprevalence in children is thought to be approximately 50%1). EBV infection may cause a spectrum of proliferative disorders ranging from self-limiting benign virus-associated hemophagocytic syndrome to lymphoid and epithelial cell malignancies.
EBV has been linked to lymphoid interstitial pneumonia (LIP) and recurrent parotid enlargement in children2). LIP causes the recruitment of various immune cells, such as plasma cells, lymphocytes, and reticuloendothelial cells, to the interstitial spaces of the lungs, and causes aberrant interactions between immune cells in immunodeficient individuals2). Microscopically, the phenotypic spectrum of pulmonary lymphoid proliferation ranges from follicular bronchitis-bronchiolitis and pulmonary lymphoid hyperplasia to malignant lymphoma3). LIP represents the benign end of the spectrum, and a relatively high proportion of LIP patients harbor latent EBV infections4). The actual incidence of lymphoma among patients with LIP is unknown. The incidence of LIP has only been reported in the immunocompromised cases5), but it is rare in children. Here, we report a patient harboring a latent EBV infection for 8 years who presented with a benign phenotype of LIP that later developed into a malignant phenotype of Burkitt's lymphoma (BL).
Case report
A 1-year-old boy made frequent visits to the local outpatient clinic due to a chronic cough and recurrent wheezing. He was tentatively diagnosed with maxillary sinusitis and bronchial asthma accompanying recurrent acute otitis media. There was no family history of chronic lung disease or asthma. At 5 years old, the boy was transferred to Asan Medical Center Children's Hospital in Seoul, Korea. He was suffering from chronic respiratory symptoms, including a chronic cough, excess sputum, and respiratory difficulties, which showed a poor response to asthma medication. Because the recurrent pneumonia was persistent, we performed high resolution computed tomography, which revealed mild bronchiectasis with peribronchial nodules (Fig. 1A-C). At 7 years old, when his respiratory symptoms were aggravated, a thoracoscopic lung biopsy was performed, which revealed LIP characterized by a dense polymorphous lymphoid infiltrate along the alveolar septa, with frequent lymphoid follicles (Fig. 1D). In situ hybridization for EBV-encoding RNA (EBER) showed frequent positive nuclear signals in small lymphocytes (Fig. 1E). The EBV genome was also detected by polymerase chain reaction (PCR). The initial EBV titer ranged from 24 copies/500 ng DNA in the blood to 204 copies/500 ng DNA and bronchoalveolar lavage (BAL) fluid. The EBV titer in the BAL waxed and waned after two cycles of acyclovir therapy and the virus remained latent. Laboratory tests performed when the boy was 5-year-old revealed the following: immunoglobulin (Ig) G, 3,660 mg/dL (normal range for the same age group, 345-1,236 mg/dL); IgM, 112 mg/dL (normal range, 43-207 mg/dL); IgA, 88.7 mg/dL (normal range, 14-159 mg/dL); C3, 122 mg/dL (normal range, 77-195 mg/dL); C4, 14.4 mg/dL (normal range, 7-40 mg/dL); and CH50, 58.2 U/mL (normal range, 60-144 U/mL). Mild hypergammaglobulinemia was also noted. Analysis of lymphocyte subsets showed the following: T cells, 50.7% (normal range, 56%-7%); CD4+ lymphocytes, 28.6% (normal range, at 2-6 years of age, 35%-51%); CD8+ lymphocytes, 20.7% (normal range, 22%-38%); natural killer cells, 30.9% (normal range, 5%-35%), and CD19+ B cells, 14.6% (normal range, 5%-23%). These results suggested normal T and B cell levels. IgG subclass analysis showed the following: IgG1, 13.7 g/L (normal range at 4-7 years of age, 2.09-9.02 g/L); IgG2, 3.78 g/L (normal range, 0.44-3.16 g/L); IgG3, 1.03 g/L (normal range, 0.11-0.95 g/L); and IgG4, 0.19 g/L (normal range, 0.008-0.82 g/L). The serum antinuclear antibody (ANA) titer was initially <1:40, but later showed a positive speckled titer of 1:80, suggesting a possible nonspecific autoimmune disorder. The patient was seronegative for human immunodeficiency virus antigen and antibodies, confirming a nonimmunocompromised state. After discharge, he was referred back to his local hospital for follow-up, and was doing relatively well, with only mild respiratory symptoms.
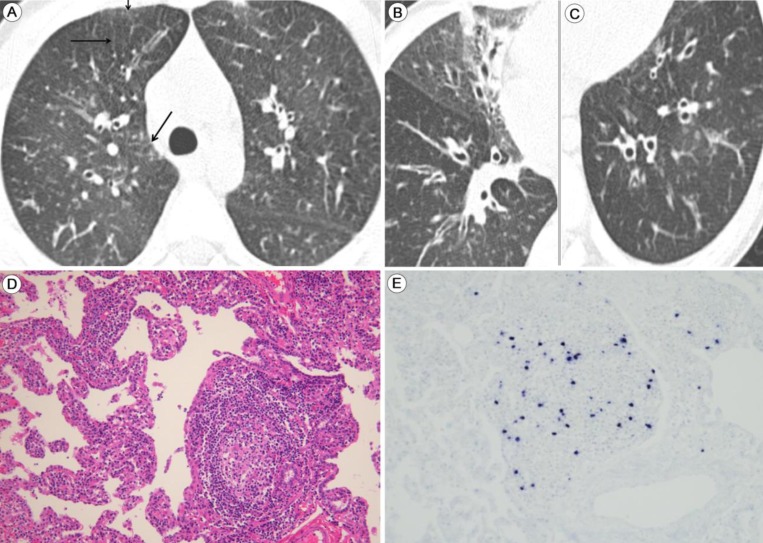
Lymphoid interstitial pneumonia (LIP) with latent Epstein-Barr virus (EBV) infection: highresolution computed tomography images and findings of the thoracoscopic biopsy. Thin-section computed tomography of the lungs taken 2 years after the first presentation of chronic recurrent respiratory symptoms. (A) Thickening of the interlobular septa (arrows) and bronchial wall. (B) Bronchial dilation with partial atelectasis in the medial segment of the right middle lobe. The thickened walls of the dilated bronchi can be observed. (C) Ectatic bronchi are seen in the left lower lobe. (D) Thoracoscopic biopsy of a lung specimen shows evidence of LIP, which is characterized by diffuse infiltration of the alveolar septa by lymphoplasmacytic cells and formation of the lymphoid follicle (H&E, ×200). (E) In situ hybridization analysis of lymph cells reveals EBV-infected cells (×200).
At 13 years old, 6 years after the diagnosis of LIP, the patient represented with intermittent abdominal pain and abdominal distension for 2 months accompanied by significant weight loss (5 kg). A physical examination revealed the following: height, 147.3 cm (3rd-5th percentile); weight, 39.4 kg (10th-25th percentile); body temperature, 37.3℃; respiratory rate, 22-30/min; blood pressure, 93/56 mmHg; and heart rate, 96 beats/min. There was no significant cervical lymphadenopathy. Chest auscultation revealed coarse breathing sounds with rales on both lower lobes in lung fields with chest subcostal muscle retraction. The heart beat was regular with no murmur. The abdomen was soft and distended. Palpation revealed multiple masses in the upper quadrant. The masses were soft with a smooth and regular margin, movable, and 15 cm in size. The patient reported tenderness in the epigastric region and left upper quadrants. There was no rebound tenderness. Splenomegaly (12 cm in size) was also noted. Peripheral blood analysis revealed the following: white blood cell, 4,200/µL (neutrophils, 58%; lymphocytes, 24%; and monocytes, 12.3%); hemoglobin, 12.9 g/dL; hematocrit, 36.9%; and platelets, 118×103/µL. An immune/complement work-up showed IgG, 2,810 mg/dL (normal range for children of the same age, 639-1,349 mg/dL); IgM, 69.3 mg/dL (normal range, 56-352 mg/dL); IgA, 771 mg/dL (normal range, 70-312 mg/dL); C3, 97.1 mg/dL (normal range, 83-177 mg/dL); C4, 23.9 mg/dL (normal range, 15-45 mg/dL); and CH50, 35.7 U/mL (normal range, 60-144 U/mL). The autoimmune profile showed that lupus erythematosus cells, anti-ds-DNA, RA factor, and anti-SSA (Ro) and anti-SSA (La) antibodies were all negative. A viral work-up showed the following: anti-EBV viral capsid antigen (EBV VCA) IgG(+), anti-EBV VCA IgM(-); anti-EBV early antigen IgM(-); and anti-EBV EBNA IgG(-). In addition, real time quantitative-PCR for EBV showed that the virus was present at 5.09 logcopies/mL (122,608 copies/mL). HIV antibody (Ab) and antigen (Ag), VDRL, cytomegalovirus (CMV) IgG, CMV IgM, hepatitis C virus Ab, hepatitis B surface Ag, hepatitis A virus Ab IgM, and PCR for mycobacterium tuberculosis were all negative, confirming a nonimmunocompromised state.
Abdomino-pelvic computed tomography (APCT) revealed multiple large infiltrating masses along the omentum, mesentery, and splenomegaly (Fig. 2A). A biopsy of the mesenteric mass revealed uniform round medium-sized tumor cells with multiple small nucleoli containing finely dispersed chromatin (Fig. 2B-F). Immunohistochemical staining showed that the tumor cells expressed CD20, CD10, and BCL6, but not BCL2, CD3, MUM-1, or cyclin D1. The Ki-67 labeling index was >95% (Fig. 2C-E). Thus, a diagnosis of BL was made by immunohistochemical confirmation. In situ hybridization for EBER showed positive nuclear signals in virtually all of the tumor cells (Fig. 2F). The ascites contained atypical lymphoid cells, a further indication of BL. A bone marrow biopsy confirmed the lack of bone marrow involvement. The patient received five cycles of chemotherapy with the CCG 5961 protocol over the following 4 months. The serum EBV viral load increased to 565,000 copies/mL, but decreased significantly thereafter. From January 2013, no systemic EBV load was detected. Radiologic findings of LIP in serial chest computed tomography revealed no interval change, although there were no residual or new masses on APCT and whole-body magnetic resonance (MR). A follow-up whole-body fluorine-18 fluoro-2-deoxyglucose positron emission tomography-computed tomography scan showed no residual hypermetabolic lesions, suggesting a complete response to the chemotherapy. The patient both tolerated and responded well to five cycles of LMB 96 (CCG5961) chemotherapy consisting of vincristine, methotrexate, cyclophosphamide, doxorubicin, cytosine arabinoside, and prednisolone and has currently been followed up at the outpatient clinic.
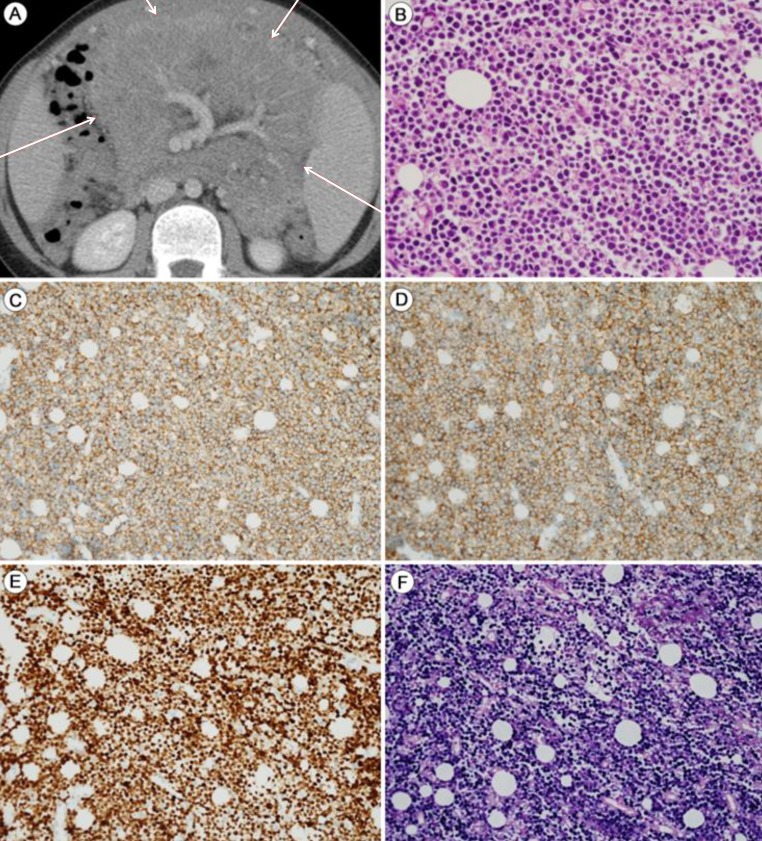
Burkitt's lymphoma (BL) with latent EBV infection: Abdominopelvic computed tomography (APCT) and mesenteric mass biopsy. The patient presented with intermittent abdominal pain and distension after harboring a latent EBV infection for 8 years. (A) APCT shows a mesenteric mass (red arrows). (B) The mesenteric mass shows evidence of Burkitt's lymphoma, with uniform round tumor cells containing multiple small nucleoli with finely dispersed chromatin (H&E, ×400). The tumor cells show strong and homogeneous expression of CD20 (C: ×200), CD10 (D: ×200), and Ki-67 (E: ×200). (F) In situ hybridization: the tumor cells are positive for EBV (×200).
Discussion
Here, we reported a case of biopsy-proven LIP in a nonimmunocompromised male patient after a long period of latent EBV infection. Six years after he was first diagnosed with LIP, and eight years with latent EBV infection, the patient presented with intermittent abdominal pain and abdominal distension along with multiple mesenteric masses. A diagnosis of BL was made. A previous study reported four EBV seropositive children with LIP and recurrent parotid enlargement preceding Non-Hodgkin's lymphoma, which was later diagnosed histologically as BL6). The patient in the present study was HIV-negative during the entire period of latent EBV infection. A recent and realistic estimate of the proportion of LIP patients that went on to develop malignant low-grade B-cell lymphoma was reported to be about 5%7).
LIP is characterized by a massive interstitial infiltration of lymphoid cells that are histologically benign and polymorphous, but have the potential to become malignant3,8,9). A previous study of four children reported three cases of LIP that preceded B-cell lymphoma6) and a recent case study reported lowgrade B-cell lymphomas with microscopic foci resembling LIP in adults3). Another study reported five instances of highgrade B-cell lymphoma arising from chronic autoimmune-associated interstitial lung disease in adults3). The differential diagnosis of LIP and malignant lymphoma is difficult because the specimen can show a mixture of chronic inflammation and malignant lymphoma cells3). Taking methodological limitations into account, immunohistochemical staining studies may not detect a small, malignant B-cell population that coexists with nonneoplastic B cells3). By contrast, our case had documented polyclonal lymphocytic traits confirmed by immunohistochemical analysis of a transbronchial lung biopsy at the time of LIP diagnosis. This evidence excludes the errors reported previously9).
Whether EBV latent infection is a necessary precursor for the development of LIP or malignant lymphomas is controversial3). The sequence of benign lymphoproliferative disease followed by the emergence of a malignant B lymphocyte clone has been reported in patients with Sjogren's syndrome8,9). In our own case, the patient showed mild hypergammaglobulinemia with ANA seropositivity, but no hypogammaglobulinemia or hypersecretion of immunoglobulins, which may be present in Sjogren's syndrome. Moreover, no malignant cells were detected by bronchoscopic cytology or in the lung specimen taken during a thoracoscopic biopsy. The fact that there were no malignant cells in the lung specimen from the thoracoscopic biopsy suggests that LIP was not intermixed with lymphoma during the initial stages of latent EBV infection. Several studies have reported cases of latent chronic EBV infection leading to BL or to the development of LIP10,11).
Our observations suggest that the chronic latent EBV infection might have caused both LIP and BL. In light of the diverse phenotypic spectrum of polyclonal B lymphoproliferative disorders, LIP is characterized as a benign low-grade de novo B-cell lymphoma, whereas BL is characterized as a malignant high grade B-cell lymphoma. It is not clear whether EBV-infected lymphocytes act as "innocent bystanders" or the "active agent"3); thus, more cases need to be examined to clarify the possible pathogenetic relationships between chronic latent EBV infection, LIP, and BL. The important clinical implications of our case highlight the variable phenotypic nature of lymphoproliferative disorders, because LIP occurred in a nonimmunocompromised individual harboring a latent EBV infection.
In conclusion, we report the case of a 14-year-old boy with an 8-year history of latent EBV infection who initially presented with LIP, which then developed into BL. This is the first documented Korean case of a latent EBV infection leading to LIP and BL in a nonimmunocompromised host.
Notes
No potential conflict of interest relevant to this article was reported.