A pediatric case of Bickerstaff's brainstem encephalitis
Article information
Abstract
Bickerstaff's brainstem encephalitis is characterized by ophthalmoplegia, ataxia, and disturbance of consciousness. It is similar to Miller Fisher syndrome, a variant of Guillain-Barre syndrome, in that they share features such as ophthalmoplegia and ataxia. The difference is that patients with Bickerstaff's brainstem encephalitis have impaired consciousness, whereas patients with Miller Fisher syndrome have alert consciousness and areflexia. Here, we report the case of a 3-year-old child who was diagnosed with Bickerstaff's brainstem encephalitis presenting typical clinical features and interesting radiological findings. The patient showed ophthalmoplegia, ataxia, and subsequent stuporous mentality. Brain magnetic resonance imaging revealed high signal intensity in the pons and cerebellum around the 4th ventricle on a T2-weighted image. He was successfully treated with intravenous immunoglobulin. Differentiation of Bickerstaff's brainstem encephalitis and Miller Fisher syndrome is often difficult because they possess many overlapping features. Brain magnetic resonance imaging may be helpful in diagnosing Bickerstaff's brainstem encephalitis, especially when lesions are definitely found.
Introduction
Bickerstaff's brainstem encephalitis was defined as ophthalmoplegia, ataxia, and impaired consciousness1). Bickerstaff's brainstem encephalitis is in the same spectrum with Miller Fisher syndrome and Guillain-Barre syndrome. They are autoimmune, postinfectious diseases and present common clinical features such as ophthalmoplegia and ataxia2). Furthermore, the etiology is regarded as similar, because they commonly show prodromal upper respiratory infection, cerebrospinal fluid (CSF) albuminocytological dissociation, and serum IgG antibody to ganglioside GQ1b3,4).
While Guillain-Barre syndrome and Miller Fisher syndrome are peripheral nervous system (PNS) diseases, Bickerstaff's brainstem encephalitis is described as a central nervous system (CNS) disease. However, the nosologic classification of Bickerstaff's brainstem encephalitis, Guillain-Barre syndrome and Miller Fisher syndrome remains uncertain. Many patients with overlap between these conditions have been reported and there is variable CNS and PNS involvement in the spectrum of these diseases4,5,6).
Here, we present the case of a 3-year-old child with typical features of Bickerstaff's brainstem encephalitis, and magnetic resonance evidence of pons and cerebellar involvement therein.
Case report
A 3-year-old boy was hospitalized because of high fever, headache, vomiting, and unsteady gait for 1 day. He had had flu-like symptoms for 3 days. There was no history of recent travelling, exposure to toxic materials, or head injury. He was fully vaccinated, and had previously been a healthy child. On examination, he showed an alert mental state and, except for a high fever, vital signs within normal limits. His pharynx was injected and neck stiffness was not observed. Both pupils were normal and responsive to light but he showed bilateral ptosis. Motor examination revealed brisk deep tendon reflexes and bilateral Babinski sign, but neither muscle weakness nor dystonia was observed. Ataxia was observed on examination. Pain and touch sensitivity were normal.
White blood cell (WBC) count in blood was 13,000 cells/µL. CSF study revealed a WBC count of 68 cells/µL, a protein level of 75 mg/dL, and a glucose level of 87 mg/dL (blood glucose was 83 mg/dL). Enterovirus was not isolated in either stool or CSF samples. Serological tests of herpes simplex virus (HSV), Epstein-Barr virus (EBV), varicella-zoster virus (VZV), and cytomegalovirus (CMV) showed negative results. A CSF culture was also negative.
We empirically administered ceftriaxone, acyclovir, dexamethasone, and mannitol to him. On the third hospital day, he showed stuporous mentality, hyperventilation and persistent fever. Furthermore, he represented sustained ptosis, upward gaze disability, slurred speech and truncal ataxia. He began to be treated in the intensive care unit.
Noncontrast brain magnetic resonance imaging (MRI) showed high signal intensity in the pons and cerebellum around the 4th ventricle on a T2-weighted image (Fig. 1). Electroencephalography (EEG) showed high amplitude slow wave activities without epileptiform discharges, suggesting encephalopathy. He was administrated immunoglobulin 1 gm/kg/day for 2 days and methylprednisolone pulse therapy. After these 2 days of treatment with immunoglobulin, he showed alert mentality and improvement of persistent downward gaze and truncal ataxia. On the 9th hospital day, he was transferred to the general ward under maintaining steroid regimen. Two weeks later, we confirmed improvement of the previously detected lesions on brain MRI (Fig. 2) and clinical symptoms including decreased mentality, ataxia and ptosis were fully recovered.
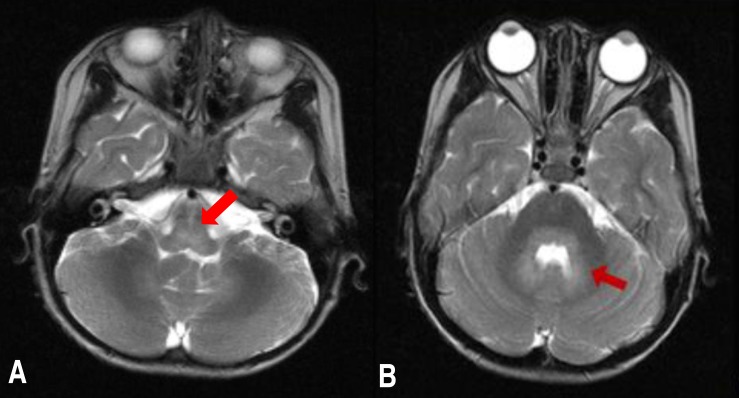
T2-weighted brain magnetic resonance imaging showing (A) hyperintensity in the pons (arrow) and (B) cerebellum (arrow).
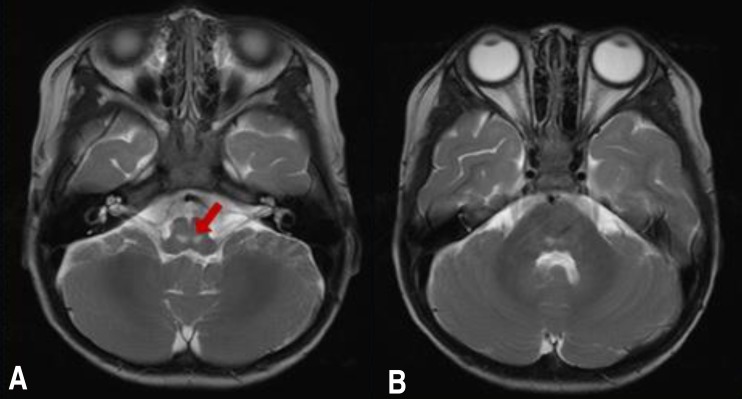
Brain magnetic resonance imaging performed after 2 weeks showed (A) only a focal T2 hyperintense lesion in the pons (arrow) and (B) improvement in the cerebellum.
On the basis of the ophthalmoplegia-ataxia-disturbance of consciousness triad with radiological evidence of CNS involvement, the diagnosis of Bickerstaff's brainstem encephalitis was made.
Discussion
Diagnostic criteria of Bickerstaff's brainstem encephalitis were described as follows4,7). "Progressive, relatively symmetric external ophthalmoplegia and ataxia by 4 weeks" and "disturbance of consciousness or hyperreflexia" are both required8). The following diseases must also be excluded: vascular disease involving the brain stem, Wernicke's encephalopathy, botulism, myasthenia gravis, brain stem tumor, pituitary apoplexy, acute disseminated encephalomyelitis, multiple sclerosis, neuro-Behcet's disease, vasculitis, lymphoma, and Creutzfeldt-Jakob disease4). In our case, the initial presentation of ataxia and ophthalmoplegia suggested the diagnosis of Miller Fisher syndrome, but brisk deep tendon reflexes and subsequent presentation of a disturbance of consciousness suggested the diagnosis of Bickerstaff's brainstem encephalitis rather than Miller Fisher syndrome. Furthermore, definite lesions on brain MRI could explain CNS involvement. In our case, the diagnosis of Bickerstaff's brainstem encephalitis was possible because of the neurological manifestations.
However, in many cases, differentiation of Bickerstaff's brainstem encephalitis and Miller Fisher syndrome is difficult because not all patients with Bickerstaff's brainstem encephalitis present abnormal brain MRI, abnormal EEG, or hyperreflexia. According to a previous report, 11% of 47 patients with Bickerstaff's brainstem encephalitis had abnormalities on brain MRI, whereas 57% of 30 patients with Bickerstaff's brainstem encephalitis had abnormalities on EEG4). Another study on 37 patients with Bickerstaff's brainstem encephalitis reported abnormalities at 23% and 50% for brain MRI and EEG, respectively9). Thus, brain MRI could not detect abnormalities in more than two-third of the patients with Bickerstaff's brainstem encephalitis. There was, however, a case report of Bickerstaff's brainstem encephalitis with normal brain MRI in which the evidence of cerebellar involvement was noted by statistical parametric mapping analysis using positron emission tomography (PET)10). As most cases of Bickerstaff's brainstem encephalitis show no abnormal lesions on brain MRI, functional imaging tools such as PET could be useful to document CNS involvement.
In fact, brain MRI findings in Bickerstaff's brainstem encephalitis have rarely been reported. Usually the findings are hyperintensity on T2-weighted images of the pons, medulla, thalamus, or cerebellum4,7). Typical lesions are noted in the brainstem and a few cases showed cerebellar lesions4,11). In our presented case, pons and cerebellar lesions could explain ophthalmoplegia, hyperreflexia, altered consciousness, and ataxia.
As mentioned above, Miller Fisher syndrome and Bickerstaff's brainstem encephalitis are supposed to have a common pathogenesis. Anti-GQ1b antibodies are commonly found in both, but more frequently in Miller Fisher syndrome4). In Miller Fisher syndrome, the anti-GQ1b antibody binds to GQ1b, expressed on the ocular nerve and primary sensory neuron; conversely, in Bickerstaff's brainstem encephalitis, it binds to GQ1b expressed on the brainstem reticular formation4,8). Therefore, the current consensus is that the two diseases are in fact part of a spectrum of autoimmune disease variably involving the PNS and CNS.
Nerve conduction study was not performed because of poor compliance; however, the patient showed no sensory loss or limb weakness despite showing ataxia. No limb weakness, but the presence of hyperreflexia and definite brain MRI lesions in the pons and cerebellum, suggested CNS rather than PNS involvement.
Unfortunately, in this case, a study of anti-GQ1b antibodies was not performed. Initially we considered viral encephalitis because the patients showed decreased mentality and CSF pleocytosis. However, clinical manifestations of this patient were different from typical viral encephalitis. He represented mental deterioration 3 days after ataxia and ophthalmoplegia. And increased CSF protein comparing to mild pleocytosis, no documented CSF bacterial or viral pathogen including EBV, HSV, and enterovirus suggested a Fisher-Bickerstaff spectrum disease. In fact, according to the epidemiological study in Japan, only 56% of the patients with Bickerstaff's brainstem encephalitis showed less than 5/mm3 of CSF cell count and 20% showed more than 50/mm3 of CSF cell count9). Moreover, dramatic response to the immunoglobulin treatment supported the evidence of Fisher-Bickerstaff spectrum disease than infective disease.
Bickerstaff's brainstem encephalitis is a rare disorder. Epidemiologic study in Korea has not been done until now. Only a few cases including a pediatric case who presented typical clinical features with positive anti-GM1 antibodies and several cases in adults with typical clinical symptoms but variety of laboratory findings were reported12,13,14). However, according to the Japanese epidemiological study, Bickerstaff's brainstem encephalitis accounted for 43% of brainstem encephalitis and anti-GQ1b antibodies were present in 75% of the patients with Bickerstaff's brainstem encephalitis9).
In conclusion, we report the case of Bickerstaff's brainstem encephalitis which is rare in childhood. The patient presented typical clinical manifestations of ataxia, ophthalmoplegia and impaired consciousness and MRI findings of definite lesions in the pons and cerebellum. Among the brain stem encephalitis, Bickerstaff's brainstem encephalitis accounts for major proportion. In cases with typical clinical manifestations, Bickerstaff's brainstem encephalitis should be considered and Anti-GQ1b antibodies may be useful in diagnosis of Bickerstaff's brainstem encephalitis.
Notes
No potential conflict of interest relevant to this article was reported.