Mechanisms of immune tolerance to allergens in children
Article information
Abstract
Because the prevalence of allergic diseases has significantly increased in recent years, understanding the causes and mechanisms of these disorders is of high importance, and intense investigations are ongoing. Current knowledge pinpoints immune tolerance mechanisms as indispensable for healthy immune response to allergens in daily life. It is evident that development and maintenance of allergens-pecific T cell tolerance is of vital importance for a healthy immune response to allergens. Such tolerance can be gained spontaneously by dose-dependent exposures to allergens in nature or by allergen-specific immunotherapy. Allergen-specific immunotherapy induces regulatory T cells with the capacity to secrete interleukin-10 and transforming growth factor-β, limits activation of effector cells of allergic inflammation (such as mast cells and basophils), and switches antibody isotype from IgE to the noninflammatory type IgG4. Although allergen-specific immunotherapy is the only method of tolerance induction in allergic individuals, several factors, such as long duration of treatment, compliance problems, and life-threatening side effects, have limited widespread applicability of this immunomodulatory treatment. To overcome these limitations, current research focuses on the introduction of allergens in more efficient and safer ways. Defining the endotypes and phenotypes of allergic diseases might provide the ability to select ideal patients, and novel biomarkers might ensure new custom-tailored therapy modalities.
Introduction
Immune tolerance is essential for maintenance of homeostasis. In the network of immune regulation, continuous stimuli-response interactions are in harmony with functional tolerance mechanisms. The numerous antigens encountered by the host in daily life, especially through mucosal surfaces, challenge the highly reactive immune system, but the stimuli normally lead to a healthy unresponsiveness, i.e., tolerance. Such unresponsiveness is essential for the well-being of the host. What happens in the state of responsiveness, as in intolerance? External antigens, or allergens, can trigger harmful hypersensitivity reactions, which might present clinically as allergic rhinitis, asthma, atopic dermatitis, food allergy, or anaphylaxis. Similar symptoms can be triggered by internal antigens. Intolerance to self-antigens leads to the development of autoimmune disorders. Excessive tolerance, however, may lead to invasion by microorganisms or parasites, or to development of cancer. Clearly, regulation of tolerance is essential for life. The best example of the paramount need for regulation of tolerance is pregnancy, in which tolerance to paternal and fetal antigens by the fetus and mother, respectively, is essential until birth.
Therapeutic induction of tolerance could restore normal immunity in conditions such as allergic and autoimmune disorders. Allergen-specific immunotherapy (SIT) is one of the best models to illustrate tolerance induction by external antigens1). Understanding the key steps in allergen-SIT might facilitate the development of novel therapeutic approaches for autoimmune disorders and cancer2).
Allergic immune response
The allergic immune response is directed against various environmental allergens. Clinical manifestations include allergic rhino-conjunctivitis, allergic asthma, atopic dermatitis, food allergy, and anaphylaxis. It has been proposed that a tendency to develop T helper type 2 (Th2) immune response is prominent in atopic individuals under the influence of genes and microenvironment3). Subsets of immune and inflammatory cells interact through cytokines. The key cytokines responsible for the allergic response include interleukin (IL) 4, IL-13, and IL-54). Specific recognition of antigenic determinants (epitopes) of allergens by T and B lymphocytes elicits the immune response5). The recognition is controlled by highly specialized antigenpresenting cells located in strategic positions, such as mucosal surfaces (gastrointestinal mucosa and airway epithelium) and the dermis. Processing and presenting allergenic epitopes to T-helper (Th) lymphocytes in the presence of relevant costimulatory cytokines, chemokines, signals, vitamins, histamine-adenosinelike small molecules, and other cells in the micro milieu shape the immune response6,7). Especially in the presence of IL-4, naive T cells activated by antigen-presenting cells differentiate into Th2 cells. In the presence of IL-4 and IL-13, class-switching in B cells promotes the synthesis of IgE antibodies. Allergen-specific IgE antibodies bind to high-affinity FcεRI receptors that are expressed on mast cells and basophils. Re-exposure to the sensitizing allergen activates mast cells and basophils to produce and release biogenic mediators (histamine, proteases, and newly generated lipid-derived mediators, such as leukotrienes and cytokines) that are responsible for the symptoms and signs of type-1 hypersensitivity allergic reactions. In the late-phase response, 6-12 hours after allergen exposure, a cell-driven process occurs, whereby eosinophils, neutrophils, basophils, T lymphocytes, and macrophages infiltrate and release additional inflammatory mediators and cytokines, perpetuating the proinflammatory response8,9). A characteristic of this phase is the heightened role of IL-5, the cytokine responsible for the activation, survival, and tissue recruitment of eosinophils. This late-phase response is thought to be responsible for the persistent, chronic signs and symptoms of allergic diseases. Continued exposure to allergen often establishes a state of chronicity10) (Fig. 1).
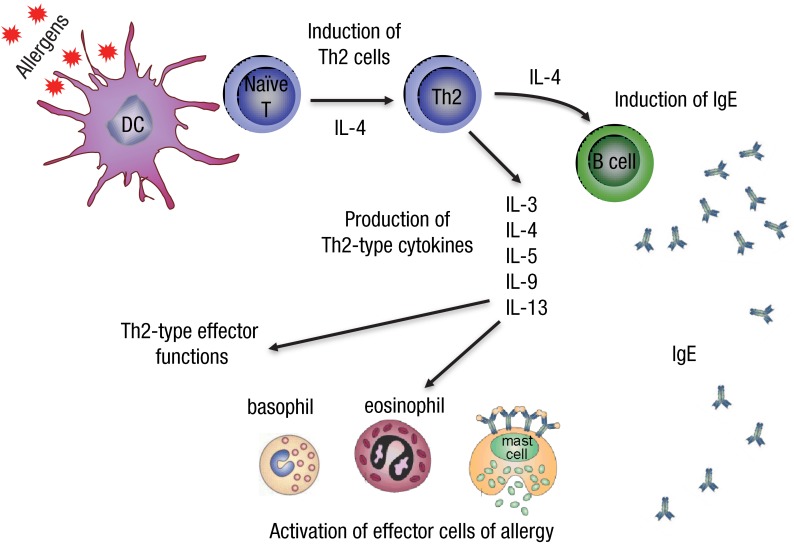
Initiation of allergy. T helper type 2 (Th2) cells are induced when dendritic cells present peptides of allergens to naive CD4+ T cells when interleukin (IL) 4 is present in the milieu. Th2 cells produce cytokines IL-3, IL-4, IL-5, IL-9, and IL-13, which are named Th2-type cytokines. B cells switch to produce IgE and bind to specific Fcε receptors on mast cells and basophils. This is known as sensitization. Upon encountering the same allergen for a second time, degranulation of mast cells and basophils takes place, leading to immediate hypersensitivity. Th2-type cytokines are important survival signals for mast cells, basophils, and eosinophils.
Allergic immune response in clinical perspectives
The allergic immune response and atopic status of children are active areas of investigation because childhood provides a period in which to study the developmental steps and natural history of allergic disorders. According to the widely known atopic march concept, exposition of atopic individuals to new antigens (possible allergens) during weaning from breast-feeding may induce clinical symptoms such as atopic dermatitis or gastrointestinal intolerability. Over time, tolerance to most food allergens develops as part of the maturing immune system. Thereafter, especially in the very early nursery years, atopic children begin to develop immune hypersensitivity to aeroallergens. Such children generally suffer from allergic rhino-sinusitis and/or asthma symptoms and require long periods of treatment regimens, which are followed by decreased quality of life11). Although allergen sensitization is known as a common cause of these disorders, substandard avoidance measures may not resolve the problem. Moreover, conventional pharmacotherapy regimens proposed by highly cited guidelines may fail to control the disease. To improve understanding of the allergic immune response, the multiple factors involved in triggering and the different phenotypes and endotypes of allergic disorders must be clarified. Future developments in this field may lead to novel customized pharmacotherapy options for cure of allergy. Several meta-analyses have delineated the impact of allergen-SIT on tolerance development. In some periods of childhood, the development of allergic disorders along with shaping the immune response depends on multifactorial stimuli. Such factors include the child's genes, microenvironment and concomitant triggering events, and age.
Regulatory T cells
Humans react to allergens in various ways. For a healthy immune response, unresponsiveness to allergens is of vital importance. Immune tolerance to allergens is the formation of long-term clinical tolerance toward allergens that is sustained by changes in memory-type and allergen-specific T and B cell responses and up-regulation of mast cell and basophil activation thresholds, with the eventual control of allergy symptoms8,12-14). Regulatory T (Treg) cells are a subset of T cells with immune regulatory properties. The naturally occurring, thymus-selected CD4+CD25+FOXP3+ Treg cells and the inducible type-1, IL-10-secreting Treg cells (Tr1 cells) are the major subsets of Treg cells. Tr1 cells are responsible for maintenance of peripheral tolerance. Forkhead box P3 (FoxP3) is the lineage-specific transcription factor for CD4+CD25+ Treg cells and has a functional master regulator role in the development of multiple subtypes of Treg cells15). Various suppressor and regulatory mechanisms have roles in the maintenance of immune homeostasis by Treg cells, which includes inhibition of the development of allergen-specific Th2 and T helper type 1 (Th1) cell responses and the direct or indirect suppression of effector cells of allergic inflammation16) (Fig. 2). Impaired allergen-specific suppressive function of CD4+CD25+ Treg cells has been demonstrated in allergic patients relative to nonallergic controls. Circulating CD4+CD25+ Treg cells in nonallergic healthy individuals have been shown to suppress proliferation of allergen-specific effector cells on exposure to allergens to a greater extent than Treg cells from sensitized individuals17).
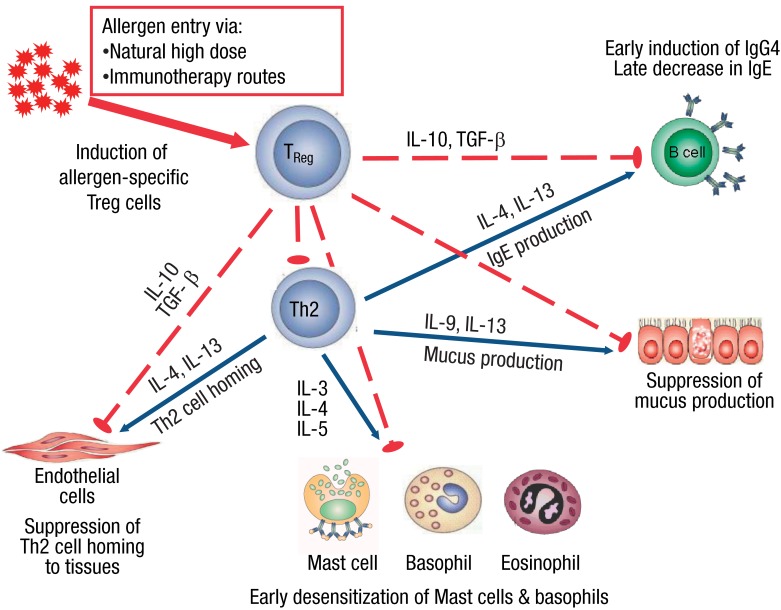
Mechanisms of tolerance to allergens. Allergen specific immunotherapy and high-dose encounters with allergens induce Treg cells, which leads to peripheral tolerance. The effector cells of allergic inflammation are regulated by regulatory and suppressive functions of Treg cells in various ways. Treg cells suppress t helper type 2 (Th2) cells and their cytokine production (interleukin [IL] 3, IL-4, IL-5, IL-9, and IL-13), which are indispensable, both for the differentiation, survival, and activity of mast cells, basophils, eosinophils, and mucus producing cells and for tissue homing of Th2 cells. IL-10 and transforming growth factor (TGF)-β suppress IgE production while inducing IgG4, which is a noninflammatory immunoglobulin isotype.
Induction of allergen-specific peripheral tolerance
Peripheral induction of T cell tolerance by allergen-specific regulatory Tr1 cells is vital to healthy immune responses to allergens18-20). The overall number of T cell numbers is controlled normally by the central tolerance mechanism. However, some T cells escape from thymic deletion and peripheral tolerance is developed in peripheral lymphoid organs. Peripheral tolerance is regulated by factors such as T cell anergy, apoptosis, Treg cells, suppressive cytokines, and antigen presenting cells (APCs)21). Studies have shown that healthy patients possess a peripheral T cell repertoire that recognizes the same T cell epitopes of allergens as allergic patients. The precise balance between the frequency of allergen-specific Th2 and Tr1 cells directed to common environmental allergens defines an allergic or a healthy status10). These findings emphasize the important contribution of Tr1 cells to the active regulation that is important in inducing and maintaining specific unresponsiveness to allergens. The unresponsiveness is primarily maintained by IL-10 and transforming growth factor (TGF)-β, which are produced by antigen-specific Treg cells. It has been suggested that both naturally occurring FoxP3+ and inducible Tr1 cells contribute to the establishment of peripheral T cell tolerance to allergens22). Establishment of clinical tolerance in humans is associated with the loss of IL-4-producing T cells and an increase of IL-10-producing antigen-specific Treg cells23). In addition to IL-10, Treg cells produce TGF-β, a suppressive cytokine. Additionally, there are changes in production of cytokines by antigen-specific T cells. A reduction in IL-4 production and an increase in IL-10 production are significant23). IL-10 and related cytokines, IL-19, IL-20, IL-22, IL-24, and IL-26, participate in T cell-mediated diseases by regulation of T cell cytokine profiles24). In addition to IL-10, expressions of costimulatory molecules CD80, CD86, and programmed cell death ligand 1 are reduced, which may down-modulate T cell immune responses25). Treg cells also strongly express CTLA-4, which inhibits T cell activation, in contrast to CD2826). TGF-β is an important suppressive cytokine that is essential for the maintenance of immunologic self-tolerance27). Conversion of naive CD4+ T cells to Treg cells requires induction of FoxP3 by TGF-β. The conversion is required for both expansion in number and suppressive capacity of Treg cells28,29).
Impact of allergen presentation
Dendritic cells (DCs) are important members of the immune system and play a pivotal role in the orchestration of immune responses by linking innate and adaptive immunity30). Circulating DCs in humans can be divided into two main groups: myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC), both of which are equipped with different repertoires of Toll-like receptors (TLRs)31,32). pDCs express TLR7 and TLR9, whereas mDCs express TLR833). Loss of allergen-specific peripheral T cell tolerance in response to TLR8-ligand (L) stimulation but not to TLR7L or TLR9L stimulation underlines the contribution of pDCs to tolerance induction34). Such important roles for pDCs had been reported in the induction and maintenance of peripheral tolerance to food and inhalant allergens in human tonsils35). In addition to DCs, TLRs also regulate B-cell responses. Stimulation of B cells with ligands for TLR3, TLR7, and TLR9 induce the generation of memory B-cells and the production of IgG1, IgA, and IgG4. Mammalian telomeric oligodeoxynucleotide (ODN) suppresses activation of B cells, production of antibodies and generation of memory cells. All of these findings support roles of TLR-triggering in the regulation of allergic responses36).
The other players of immunity
Subsets of antigen-presenting cells, such as DCs and B-cells, that are capable of producing IL-10 were shown to contribute to the suppressive effects of IL-1037,38). Natural killer (NK) cells are important factors in innate immunity. Circulating NK cells retain effector subsets, such as NK1 and NK2, with distinct cytokine profiles, similar to Th1 and Th2-cells. These cells are claimed to display different inflammatory properties39). Recent studies have revealed a subset of NK cells that produce IL-10 and suppress allergen-specific T cell responses40). Recently, a subset of B cells that are capable of producing IL-10, expressing IgG4, and suppressing allergen-specific CD4+ T cell proliferation has been demonstrated and named as B-regulatory 1 cells41). Suppression by IL-10 occurs in various ways. T cells are suppressed by blocking CD2, CD28, and inducible costimulator (ICOS) costimulatory signals by IL-10. The mechanism employs the Src homology 2 domain-containing tyrosine phosphatase (SHP-1) which rapidly binds to CD28 and ICOS and de-phosphorylates them42,43). IL-10 also inhibits activated monocytes and macrophages which is revealed by suppression of costimulatory molecules on these cells and by down-regulation of MHC class II molecules and down-regulation of antigen-presenting cell capacity44,45). It has been shown that expression of the SOCS3 gene was induced by IL-10, possibly due to the inhibition of interferon (IFN)-γ-induced tyrosine phosphorylation of signal transducer and activator 146).
Antibody responses during tolerance development
Induction of peripheral tolerance also induces changes in antibody isotypes. Serum allergen-specific IgE levels decrease gradually, while allergen-specific IgG4 blocking antibodies increase during allergen-SIT9,47,48). In contrast to T cells, B cell responses to antigenic stimuli are not fully suppressed. They switch to produce IgG4 instead of IgE47). IL-10 contributes to the regulation of specific antibody isotypes to move toward a noninflammatory phenotype49,50). Important blocking effects of IgG4 are maintained by its ability to compete with the Fcε receptors on IgE for binding antigens. Because IgE is expressed on the surface of mast cells and basophils, the competition for binding antigens limits effector cell activation and degranulation51). In addition, blocking antibodies can prevent the activation of CD4+ cells by inhibiting CD23-mediated IgE-facilitated presentation of antigens52).
Immune regulatory role of histamine
Histamine has functional roles in immune regulation through four distinct histamine receptors (HR). Among these, HR2, which is relatively highly expressed on Th2 cells, induces Treg cells and supports development of antigen-specific peripheral tolerance. Up-regulation of HR2 increases IL-10 levels, suppresses T cell stimulation, and enhances the suppressive action of TGF-β on T cells. HR4 modulates migration of eosinophils and recruitment of mast cells and is involved in DC activation and T cell differentiation7,53).
Conventional routes for immunotherapy; subcutaneous and sublingual ways
Recent studies have extensively investigated mechanisms of immune tolerance induction. The therapeutic induction of immune tolerance through either allergen-SIT or spontaneous induction, such as in beekeepers or cat owners, is highly important to the field. Complex mechanisms have been proposed to explain establishment of peripheral tolerance50,54,55). SIT is known to be the only available curative treatment of allergic diseases. Peripheral tolerance induction by the generation of allergen-specific Treg cells, which have suppressive proliferative and cytokine response capabilities, is the consequence of SIT19,49). Subcutaneous immunotherapy and sublingual immunotherapy (SLIT) routes are primary administrative routes for immunotherapy. In addition to bee-venom injection immunotherapy, both routes are successfully used for pediatric and adult allergic rhinitis and asthma patients56,57). Increased production of IFN-γ following SLIT has been correlated with the success of immunotherapy58).
Peripheral tolerance can also be established as a consequence of natural, high-dose allergen exposure. In healthy nonallergic beekeepers and cat owners55,59), Treg cells specific for the relevant major allergens become the major T cell subset of these individuals by utilizing multiple suppressive mechanisms, such as secreted cytokines IL-10 and TGF-β and surface molecules CTLA-4 and PD-1. High-dose exposure models such as beekeepers enhance our understanding of the nature of Treg responses in immune tolerance induction22).
Novel therapeutic approaches of allergen-SIT
The side effects and long duration of treatment in allergen-SIT are undeniable negative impacts on the use of this strategic treatment. Improvements in ease of administration and safety may expand application to indications such as atopic dermatitis, food allergies, and large local bee sting reactions. Promising developments in the field of allergen-SIT include modified allergens and new routes of administration. Knowledge of the influence of IgE-facilitated antigen presentation in allergen-specific Th2 responses have led to investigation of methods to generate non-IgE-binding allergens60). Utilizing genetic engineering methods, two major bee venom allergens, phospholipase A2 and hyaluronidase, have been fused in a manner that removes B-cell epitopes in order to prevent IgE cross-linking and preserves T cell epitopes due to conformational changes of the antibody. The novel fusion protein Api M (1/2) abolishes IgE reactivity, reduces basophil degranulation, and produces type-1 skin reactivity61). In addition to allergen modification, routes of administration are also under intense investigation. A novel method to introduce the allergen in a more efficient way was recently described. Modular antigen translocating (MAT) molecules present the allergen to the major histocompatibility class-II pathway intracellularly, which enhances antigen presentation. The rapid translocation of the MAT-fused allergens in the cytoplasm provides a significant advantage. Intracytoplasmic accumulation of the MAT-fused allergens can induce stronger proliferation responses to the corresponding allergens, even at 10.100 times lower concentrations. Cytokine responses in PBMC cultures revealed marked increases in IL-10 and IFN-γ secretion and decreases in IL-4 and IL-5 responses compared with those induced by the corresponding recombinant allergen62).
As a promising route, intralymphatic allergen administration under ultrasound guidance can induce tolerance with three injections, which provides a significant advantage over classical multiple subcutaneous injections. Recently, a randomized double-blind safety and efficacy study of intralymphatic immunotherapy (ILIT) with the recombinant major cat dander allergen Fel d 1-MAT molecule was reported. The increase in nasal reactivity to the allergen in the ILIT group was pronounced compared with the placebo group. In addition, it was shown that in the ILIT group, T regulatory cell response was induced along with an IL-10 response and increased cat dander-specific IgG4 levels63). In another double-blind, placebo-controlled study, clinical improvement in nasal allergy symptoms was reported in patients with allergic rhinitis who had been treated with three intralymphatic injections of birch or grass pollen64).
Similarly, epicutaneous immunotherapy (EPIT) is another novel approach in which highly immunogenic skin is the target. Although the first successful intervention was in a horse dander allergic asthmatic patient in 192165), this route has recently regained attention. In a recent placebo-controlled, double-blind study, the safety and efficacy of EPIT in grass pollen allergic rhinitis patients was shown in a dose-dependent manner after only six patches66). In another prospective double-blind trial in grass pollen allergic children, transcutaneous administration of the allergens was shown to be effective in reducing symptoms and the use of antihistamines67). Progress in EPIT is promising because this method can be a noninvasive alternative to immunotherapy in patients with injection-phobia, especially in children.
Loss of peripheral tolerance due to inflammation
Allergen-specific tolerance is maintained long-term, once established. However, the observation of allergic exacerbations followed by infections, especially viral infections, has triggered studies to investigate the breakage of peripheral tolerance. Recent findings revealed that activation of innate immunity by specific activators condition specific adaptive immune responses to allergens. IL-1β and IL-6 cytokines, with proinflammatory properties, and the triggering of TLR4 and TLR8, which recognize microbial particles as danger signals, have been shown to lead to proliferation of allergen-specific CD4+ T cells in peripheral blood of normally unresponsive individuals by various pathways, such as forming an inflammatory milieu, potentiating mDCs, or rendering T cells unresponsive to Treg suppression34). These findings may shed light on how healthy humans develop allergic diseases upon encountering microbes or inflammatory conditions.
Future perspectives
Allergen-SIT remains to be the major procedure for longterm management of allergic disorders. Short falls in strict allergen-avoidance measures and failures of sustained clinical responses to pharmacotherapies increase the demand for more efficient and safer allergen immunotherapy regimens. With increasing understanding of the roles of cytokines, chemokines, pathways, networks, and cellular interactions, novel modulated administration routes are being tested and allergens are being developed for more successful immunotherapy regimens. However, the new immunotherapy regimens show variable clinical responses in allergic individuals. This might be due to different endotypes and phenotypes of allergic diseases. Discovery of biomarkers to identify these endotypes and phenotypes may provide optimum patient selection for relevant immunotherapy regimen. Definition of these phenotypes and endotypes will enable selection of the best patient for more efficient treatment68). The field of allergen-SIT will expand with answers to the key questions: "In which patient?", "Which route?", "Which allergen?", and "What would be the duration for immunotherapy?" Therefore, the importance of establishing data to describe the differences between endotypes and phenotypes is crucial. Mechanistic studies designed to improve understanding of diseases and risk factors and to lead to improved diagnosis and therapy should be performed69). Characterization and increased understanding of the underlying disease mechanisms in allergic diseases might require the use of individualized combined applications of immune response modifiers with allergen-SIT.
Acknowledgments
The author's laboratory is supported by the Swiss National Science Foundation grant 320030-132899 and Christine Kühne-Center for Allergy Research and Education (CK-CARE).
Notes
No potential conflict of interest relevant to this article was reported.