Association of wheezing phenotypes with fractional exhaled nitric oxide in children
Article information
Abstract
Asthma comprises a heterogeneous group of disorders characterized by airway inflammation, airway obstruction, and airway hyperresponsiveness (AHR). Airway inflammation, which induces AHR and recurrence of asthma, is the main pathophysiology of asthma. The fractional exhaled nitric oxide (FeNO) level is a noninvasive, reproducible measurement of eosinophilic airway inflammation that is easy to perform in young children. As airway inflammation precedes asthma attacks and airway obstruction, elevated FeNO levels may be useful as predictive markers for risk of recurrence of asthma. This review discusses FeNO measurements among early-childhood wheezing phenotypes that have been identified in large-scale longitudinal studies. These wheezing phenotypes are classified into three to six categories based on the onset and persistence of wheezing from birth to later childhood. Each phenotype has characteristic findings for atopic sensitization, lung function, AHR, or FeNO. For example, in one birth cohort study, children with asthma and persistent wheezing at 7 years had higher FeNO levels at 4 years compared to children without wheezing, which suggested that FeNO could be a predictive marker for later development of asthma. Preschool-aged children with recurrent wheezing and stringent asthma predictive indices also had higher FeNO levels in the first 4 years of life compared to children with wheezing and loose indices or children with no wheeze, suggesting that FeNO measurements may provide an additional parameter for predicting persistent wheezing in preschool children. Additional large-scale longitudinal studies are required to establish cutoff levels for FeNO as a risk factor for persistent asthma.
Introduction
Asthma is a heterogeneous group of disorders associated with recurrent episodes of wheezing, coughing, chest tightness, and shortness of breath. Although recurrent wheezing is the most common symptom suggesting asthma, not all children with wheezing are asthmatic, and only a small percentage of infants with wheezing will develop asthma in later childhood1). Early intervention and treatment of asthma is important to improve the prognosis and decrease irreversible airway remodeling. Chronic airway inflammation is present even in children with mild asthma and leads to recurrent asthma attacks. Therefore, identification of a predictive marker for asthma among preschool children might help in early diagnosis and management of asthma. Several large-scale birth cohort studies have suggested wheezing phenotypes that may help to predict the development of asthma in later life2,3).
Airway inflammation, airway hyperresponsiveness (AHR), and variable airway obstruction are key to the pathophysiology of asthma. Airway inflammation is present even during asymptomatic periods of asthma and may precede the onset of asthma4). Airway inflammation also induces recurrent asthma attacks and leads to airway remodeling. Therefore, early detection of airway inflammation is a cornerstone of asthma therapy, and while objective measurement for AHR or airway obstruction have made advances in clinical practice, assessment of airway inflammation using bronchial biopsy or bronchoalveolar lavage remains too invasive for children.
The fractional exhaled nitric oxide (FeNO) level is a noninvasive, easy, and reproducible measurement of eosinophilic airway inflammation5). In recent studies, the FeNO level has been shown to be useful in the diagnosis of asthma as well as in monitoring steroid-responsiveness of asthma6). FeNO levels correlate well to both AHR and atopy in children7).
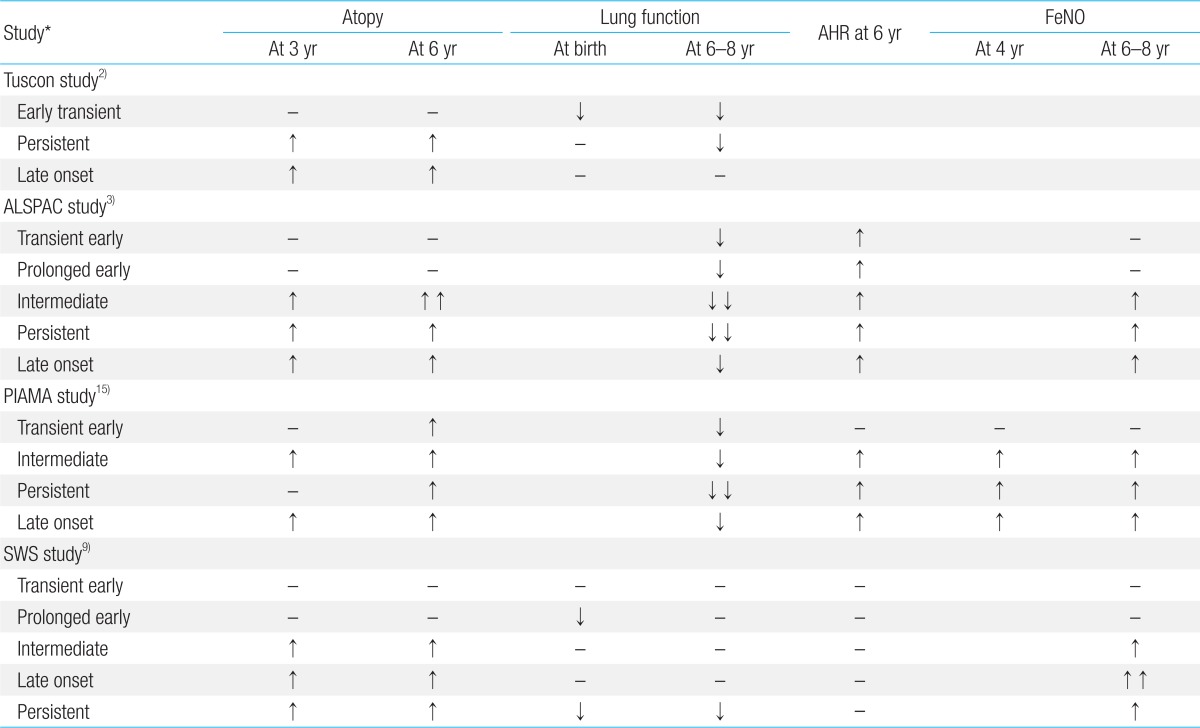
Several longitudinal cohort studies have been performed in order to identify clinical and laboratory characteristics of wheezing phenotypes that can help to distinguish children who will develop asthma from those who have transient symptoms only. FeNO levels can be assessed easily in preschool children with recurrent wheezing and may be useful as a predictive biomarker for persistence of asthma into later childhood. Only a few studies have shown an association of high FeNO levels in persistent wheezing phenotypes when compared to transient wheezing phenotypes in preschool children. This review will focus on the association of lung function tests, atopic sensitization, and FeNO levels with wheezing phenotypes in early childhood and summarize the results of several large birth cohort studies (Table 1).
Wheezing phenotypes in early childhood
Several large longitudinal cohort studies have classified wheezing phenotypes in preschool and young children according to the onset and persistence of wheezing. Based on these qualities, the Tucson Children's Respiratory Study first classified wheezing among 826 children from birth to 6 years into four phenotypes2): no wheezing (51.5%); early transient wheezing (19.9%); persistent wheezing (13.7%); and late onset wheezing (15.0%). The early transient wheezing group had a high prevalence of wheezing before the age of 3 years that resolved by the age of 6 years, while the persistent and late-onset wheezing groups had persistent wheezing even at 6 years old. The prevalence of disease ever diagnosed as asthma at 6 years was 46.0% in children with persistent wheeze compared to 22.5% in children with late onset wheeze.
Later, the Avon Longitudinal Study of Parents and Children (ALSPAC) reclassified wheezing into six phenotypes using latent class analysis in 6,265 children from birth to 7 years3). These were: no/infrequent wheezing (59%); transient early wheezing (16%); prolonged early wheezing (9%); intermediate onset wheezing (3%); late onset wheezing (16%); and persistent wheezing (7%). The group with intermediate onset wheezing had a low prevalence of wheeze up to 18 months and high prevalence from 42 months, while the prolonged early wheeze group had a peak prevalence of wheezing at 30 months with declining prevalence from 69 months. The ALSPAC identified a higher prevalence of asthma by 7 years in the intermediate onset wheeze group compared to the late onset wheeze group.
The Prevention of Infant Asthma and Mite Allergy (PIAMA) cohort study identified five phenotypes of wheezing among children after replicating the ALSPAC process with 2,810 patients from birth up to 8 years of age8): never/infrequent (75.0%); transient early (16.7%); intermediate onset (3.1%); late onset (1.7%); and persistent (3.5%). Transient early wheezing in the PIAMA study was characterized by a high prevalence of wheezing at 12 months with a decline thereafter to a low prevalence at 84 months. This phenotype represented a combination of the prolonged early wheezing and the transient early wheezing phenotypes described in the ALSPAC study. In the PIAMA model, persistent, late onset, and intermediate onset wheeze were strongly associated with asthma at 8 years of age8).
The Southampton Women's Survey (SWS) successfully validated the ALSPAC phenotypes using 6-year follow-up data for longitudinal lung function testing and atopic sensitization among 940 children from birth to 6 years9). The SWS included infant lung function results at 6 weeks; spirometry, FeNO, and methacholine challenge results at 6 years; and skin prick testing at 12 months, 3 years, and 6 years. The SWS cohort study classified children by ALSPAC and Tucson phenotypes according to wheezing data at 6 and 12 months and at 2, 3, and 6 years. Prevalence of the ALSPAC wheeze phenotypes was never, 40.3%; transient early, 17.4%; prolonged early, 27.6%; intermediate onset, 6.2%; late onset, 2.2%; and persistent, 6.4%, and that of the Tucson phenotypes was never, 40.3%; transient early, 45.0%; late onset, 2.2%; and persistent, 12.5%.
Lung function and atopic sensitization according to wheezing phenotype
The Tucson study determined that children with transient early wheeze had diminished airway function at birth that was still present at the age of 6 years and were nonatopic, while children with persistent wheeze phenotype had normal lung function at birth that was diminished at 6 years of age and had atopic sensitization2). Those with late onset wheeze had normal lung function both at birth and at 6 years with a higher prevalence of atopic sensitization at 6 years compared to those with the never wheeze phenotype. This was supported by the Manchester Asthma and Allergy Study (MAAS)10), a population-based birth cohort study that measured specific airway resistance at ages 3 and 5 years and analyzed the association of lung function with the Tucson wheeze phenotypes. The MAAS study found that persistent wheeze and transient early wheeze were associated with poor lung function at both 3 years and 5 years, while preschool age children with late onset wheeze had normal lung function. In addition, persistent wheeze was associated with steeply increasing airway resistance at 5 years compared to 3 years. On the other hand, the Copenhagen Prospective Studies on Asthma and Childhood cohort study found that children with asthma at 7 years of age had lung function deficits during the neonatal period that progressed during early childhood; the neonates also had AHR11).
The ALSPAC study assessed atopic sensitization, spirometric lung function, and AHR at 7 to 9 years of age. The study determined that intermediate onset wheeze had the strongest associations with atopy, lung function deficit, and AHR at 7 to 9 years, followed by late onset wheeze and persistent wheeze, respectively. Transient and prolonged early wheeze did not show associations with atopy and had weak associations with AHR3).
The PIAMA study found that airway resistance measured at 4 years with the interrupter technique was higher in children with persistent wheeze than in those with never wheeze and early wheeze phenotypes12).
In the SWS study, children with intermediate onset wheeze showed earlier atopic sensitization, at 1 year, while those with persistent and late onset wheeze had later atopic sensitization at 3 and 6 years. Those with intermediate onset wheeze showed no lung function deficit at 6 weeks and 6 months, while persistent wheeze was associated with reduced lung function at 6 weeks and 6 years. Children with prolonged early wheeze were nonatopic, but had lung function deficits as infants and at 6 years, while the transient early wheeze group showed no decrease in lung function at 6 weeks or at 6 years9).
In the PIAMA study, atopic sensitization at 4 years was suggested as a predictive marker for asthma or wheezing at 8 years13), and a positive specific IgE to any aeroallergen was strongly associated with wheezing at 8 years12). In most cohort studies, wheezing at 6 to 8 years of age has been strongly associated with atopic sensitization and AHR, and taken together, it appears that atopic sensitization in early childhood may be a very important factor in the persistence of asthma among certain children with AHR.
FeNO levels according to wheezing phenotype
FeNO is a simple and easy measurement, and an objective marker for airway inflammation with excellent reproducibility. FeNO has been shown to be superior to lung function testing by impulse oscillometry (IOS) in preschool children, with a sensitivity of 86% and a specificity of 92% to distinguish between children with probable asthma and healthy controls14).
The first FeNO measurements for preschool children participating in a birth cohort study were reported in the PIAMA study15). The children were divided into Tucson wheezing phenotypes2) at 4 years of age based on wheezing episodes before the first 3 years of life and in the 4th year of life. Although FeNO levels were higher in children with atopy or with physician-diagnosed asthma, there was no association between FeNO levels and wheezing phenotypes15). However, when the authors recategorized children into the five PIAMA8) wheezing phenotypes at 8 years of age and reassessed the association of FeNO levels at 4 years and 8 years, they found that the FeNO levels at 4 years were higher in children with intermediate onset and persistent wheeze compared to those with never wheeze and transient early wheeze phenotypes16). This finding is in line with our study showing higher FeNO levels in preschool children with persistent and late onset wheeze compared to those with no wheeze7). Similarly, the SWS study identified higher FeNO levels at 6 years in children with intermediate onset, late onset, and persistent wheeze9).
Atopy is an important factor for high FeNO levels in children with persistent asthma. In the PIAMA study, FeNO levels at 8 years showed a significant increase in intermediate onset, persistent, and late onset wheeze phenotypes compared to never/infrequent wheeze only in children who had atopic sensitization at 8 years. We also showed that children with persistent wheeze with atopy and AHR had higher FeNO values compared to those with persistent wheeze without atopy or AHR7). Interestingly, in our study, neither spirometry nor IOS showed any difference between persistent wheeze and nonwheeze in preschool children7). This implies that airway inflammation is present even during a period when there is no airway obstruction in preschool children with persistent asthma.
The possibility that FeNO levels are a risk factor for later asthma development has also been shown in a cross-sectional study with preschool children aged 3 to 47 months17). This study categorized subgroups using the asthma predictive index (API), which is a clinical index that defines the risk of asthma at 6 years in young children with recurrent wheeze18). Preschool children with recurrent wheeze and a stringent API had higher FeNO levels compared to both children with recurrent wheezing with a loose API and children with no wheeze17). This suggests that preschool children with recurrent wheeze and a higher risk of asthma at 6 years have higher FeNO levels in the first 4 years of life, and it was supported by another study from PIAMA that showed that higher FeNO levels at 4 years were associated with a physician diagnosis of asthma at 7 years and with a higher prevalence of wheezing between 5 and 8 years of age13). Atopic sensitization at 4 years was also associated with physician diagnosis of asthma up to the age of 8 years. Recently, a new API including high FeNO values as a major criterion was suggested in a cohort study of 391 high-risk preschool children aged 3-47 months19). In this study, the median FeNO was significantly increased in preschool children who developed asthma at school age compared to those who did not develop asthma (10.5 ppb vs. 7.4 ppb). In new API, FeNO>10 ppb was added as a major criterion, and blood eosinophilia ≥4% was removed from a minor criterion. The authors compared the new API to the classical API, and concluded the diagnostic performance was similar. The new API could identify a risk for later asthma development without blood sampling so that it could replace the classical API for the prediction of school-age asthma in preschool children when clinically relevant.
There are several considerations for use of FeNO testing in clinical practice for young children. The FeNO level is influenced by factors such as height, age, atopic status, smoking, infection, medications, measurement technique, exhalation flow rate, and the NO analyzer used. Furthermore, there are several methods of FeNO measurement in children, which are categorized as on-line and off-line methods with constant exhaled flow or tidal breathing4,20). Measurements with a constant exhaled flow rate require the patient's cooperation and the ability to breathe at a constant flow of 50 mL/sec. Therefore, this method is difficult for children under 4 years old, and methods of collecting exhaled NO in balloons during spontaneous breathing have been introduced for these children4,21,22,23,24). During quiet breathing, exhaled gas is collected into balloons and FeNO concentration is measured with an NO analyzer. The American Thoracic Society (ATS) guidelines recommend that age has to be considered as a factor influencing FeNO results in children younger than 12 years of age and that FeNO levels >35 ppb are likely, and FeNO levels <20 ppb are unlikely, to be used as cutoff level for eosinophilic inflammation in symptomatic children25). In healthy children aged 1 year to 4 years, a geometric mean 7.1 ppb (95% confidence interval, 2.8-11.5) was suggested as a reference value of exhaled NO using off-line tidal breathing methods23). As FeNO is also highly affected by atopic status, it is necessary to determine cutoff values of FeNO that are stratified for atopy and to validate the cutoff points according to height and age. However, a recent study in healthy elementary school children showed no association between age and FeNO values, while total IgE, percent blood eosinophils, and height were positively associated with FeNO levels24). They demonstrated FeNO reference equations using multiple linear regression analysis with considering the variables of height, total IgE, and eosinophil percent. Finally, although FeNO measurement protocols were published by the European Respiratory Society/ATS in 2005, there may still be different values depending on the detection methods. Electrochemical sensor devices have detection levels that are 4 to 10 ppb lower than those of chemiluminescent devices26).
conclusions
There are different wheezing phenotypes in children, but a predictive marker for persistence of wheezing has yet to be clarified. Although AHR or lung function deficits have shown conflicting results among studies, most studies have suggested atopic sensitization as well as elevated FeNO levels as a risk factor for the persistence of wheezing. FeNO measurement may serve as an additional parameter for predicting persistent wheezing in preschool children. Additional large-scale longitudinal studies are required to establish cutoff values of FeNO as a risk factor for persistent asthma.
Notes
No potential conflict of interest relevant to this article was reported.