Plasmaphresis therapy for pulmonary hemorrhage in a pediatric patient with IgA nephropathy
Article information
Abstract
IgA nephropathy usually presents as asymptomatic microscopic hematuria or proteinuria or episodic gross hematuria after upper respiratory infection. It is an uncommon cause of end-stage renal failure in childhood. Pulmonary hemorrhage associated with IgA nephropathy is an unusual life-threatening manifestation in pediatric patients and is usually treated with aggressive immunosuppression. Pulmonary hemorrhage and renal failure usually occur concurrently, and the pulmonary manifestation is believed to be caused by the same immune process. We present the case of a 14-year-old patient with IgA nephropathy who had already progressed to end-stage renal failure in spite of immunosuppression and presented with pulmonary hemorrhage during oral prednisone treatment. His lung disease was comparable to diffuse alveolar hemorrhage and was successfully treated with plasmapheresis followed by oral prednisone. This case suggests that pulmonary hemorrhage may develop independently of renal manifestation, and that plasmapheresis should be considered as adjunctive therapy to immunosuppressive medication for treating IgA nephropathy with pulmonary hemorrhage.
Introduction
Pulmonary renal syndrome is an unusual, life-threatening manifestation that is characterized by a combination of diffuse alveolar hemorrhage and glomerulonephritis1). Common causes of this manifestation in childhood include Wegener's granulomatosis, pauci-immune vasculitis, Goodpasture syndrome and lupus nephritis1). Rare causes include poststreptococcal glomerulonephritis, cryoglobulinemia, rheumatoid arthritis, scleroderma, polymyositis, Henoch-Schönlein purpura, IgA nephropathy, Behcet disease, antiphospholipid syndrome, thrombotic thrombocytopenic purpura, neoplasm, and medication1).
Within this, IgA nephropathy is an extremely rare cause of pulmonary renal syndrome for which only 12 cases, including 2 pediatric cases, have been reported2345678). The pathogenesis of pulmonary hemorrhage in IgA nephropathy is poorly understood. Given that the alveoli and glomeruli share a vascular histology of tortuous capillaries and similar antigens, circulating antibodies have been suggested as the mechanism of pulmonary renal syndrome in IgA nephropathy39). However, circulating antibodies or IgA staining in lung biopsy has not been identified345). A few cases suggest that pauci-immune pathology attack alveolar capillaries and glomeruli3). As renal and pulmonary manifestations usually occur concurrently, and given that these are known to be caused by same underlying immune process, pulmonary hemorrhage is an exceptional situation in a patient who has already progressed to end-stage renal failure (ESRF)245678).
The pulmonary manifestations associated with IgA nephropathy cause above 50% mortality and early treatment is necessary2357). Although immunosuppressants such as steroids have been used, there are as yet no evidence-based guidelines for treatment. However, previously reported cases have suggested that outcomes can be improved with aggressive immunosuppression therapy2348). A few cases showed that plasmapheresis can be used as an adjuvant therapy to immunosuppressive medication in IgA nephropathy with pulmonary hemorrhage12). We describe a pediatric patient with pulmonary hemorrhage associated with IgA nephropathy which progressed to ESRF in spite of immunosuppressant treatment and highlight the use of plasmapheresis as adjunctive therapy to steroids.
Case report
A 14-year-old male patient presented with hemoptysis and progressive dyspnea. He had first presented with proteinuria and microscopic hematuria four years earlier at school screening and was diagnosed as having IgA nephropathy by renal biopsy. Renal biopsy at that time had shown diffuse mesangial proliferative glomerulonephritis with positive immunofluorescence staining for IgA. After the diagnosis of IgA nephropathy, he took medication including oral prednisone, azathioprine, cyclosporine A, and angiotensin-converting-enzyme inhibitor. However, the proteinuria persisted and renal function deteriorated. One month prior to admission, he presented with upper respiratory tract infection and received oral antibiotics for one week. At that time, he had a serum creatinine level of 1.78 mg/dL and random urine protein/Cr 12.92 mg/mg Cr. With supportive management, his respiratory symptoms resolved.
He visited our outpatient department for regular follow-up. He showed no specific symptoms and denied a history of herbal medication. Blood pressure was 143/97 mmHg. Laboratory testing revealed urea of 41.3mg/dL, creatinine of 6.78mg/dL, and hemoglobin of 9.5 g/dL. He had normal platelet and white blood cell counts. Serum C3 and C4 levels were normal. Serum antinuclear antibody, antiglomerular basement membrane antibody, and antineutrophilic cytoplasmic antibody (ANCA) were all negative. Urinalysis revealed persistent albuminuria and hematuria. There was no lung lesion visible on x-ray and kidney ultrasonography showed diffusely increased parenchymal echogenicity in both kidneys.
After admission, the azotemia did not improved despite intravenous hydration treatment. A kidney biopsy was performed. Light microscopy analysis revealed 16 cellular crescents, 5 fibrocellular crescents and 18 global sclerosis among 45 glomeruli (Fig. 1A). Mesangial proliferation was also detected. Tubules revealed mild atrophy and subacute damage with interstitial fibrosis and moderate mononuclear infiltration. The immunofluorescence findings showed mesangial staining for IgA and C3 (Fig. 1B). Electron microscopy revealed severe effacement of the epithelial foot processes and mesangial immune deposits (Fig. 1C). The kidney biopsy results suggested rapidly progressive glomerulonephritis due to aggravation of IgA nephropathy. Treatment was initiated with intravenous methylprednisolone pulse therapy and oral cyclophosphamide. However, his disease progressed to ESRF and he received hemodialysis three times per week. After 1 month of admission, the dialysis modality was changed to night intermittent peritoneal dialysis.
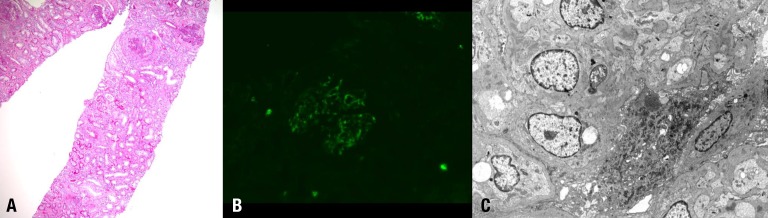
Histologic findings of the kidney. (A) Renal biopsy showed the following findings: cellular crescent, mesangial proliferation, tubular atrophy and interstitial fibrosis (H&E, ×100). (B) Immunofluorescence microscopy of glomeruli demonstrated mesangial staining for IgA (IgA antibody, ×100). (C) Ultrastructural examination of the glomeruli showed severe effacement of epithelial foot processes and mesangial immune deposits (×100).
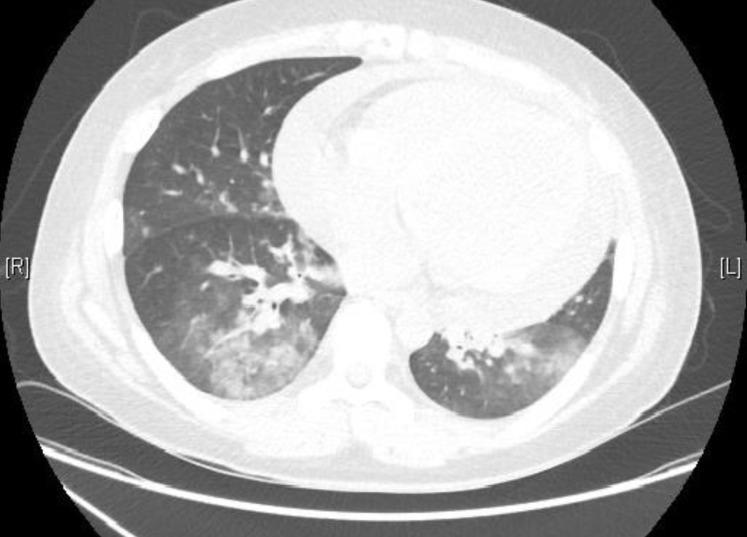
Two months after admission, hemoptysis and progressive dyspnea suddenly developed with oral prednisolone and cyclophosphamide. He was clinically euvolemic and no skin lesion was seen. Auscultatory examination of the chest revealed bilateral coarse crepitations. Chest x-ray showed bilateral pulmonary infiltration and chest computed tomography showed pulmonary hemorrhagic lesions on both lower lung fields (Fig. 2). He had no history of pulmonary symptoms or reported abnormalities on chest x-ray. Our patient was a nonsmoker with no known exposure to nonprescribed medication. There were no gastrointestinal, joint or skin symptoms. Extensive microbiologic screening results were all negative. In the laboratory findings, there was no risk of bleeding tendency. A prothrombin time international normalized ratio was 1.02, activated partial thromboplastin time 38.7 seconds, and platelet counts 130,000/mm3. With the peritoneal dialysis, urine protein was 1.3 g/day, and glomerular filtration rate 3.1 mL/min/1.73 m2. Repeated tests for serum antinuclear antibody, antiglomerular basement membrane antibody, and ANCA were also all negative. Pulse oximetry was below 90% on a FiO2 of 40%. He was moved to the intensive care unit due to hypoxia, which was treated with mask oxygen supplementation. He received continuous renal replacement therapy (CRRT) and intravenous antibiotics and antifungal agent. However, there was no clinical response to the antibiotics, antifungal agent or CRRT. Heavy hemosiderin-laden macrophages were seen by bronchoscopy. Cultures from the bronchoalveolar lavage were negative for microorganisms. This finding was suggestive of diffuse alveolar hemorrhage. The clinical manifestations and bronchoscopy findings suggested a pulmonary renal syndrome secondary to IgA nephropathy. The patient received daily plasmapheresis with albumin and intravenous methylprednisolone at 1 mg/kg/day and the respiratory symptoms and hypoxia showed rapid resolution after 3 plasmapheresis treatments. The chest x-ray findings were also improved. The patient was discharged with an oral prednisolone dose of 0.5 mg/kg/day and weekly plasmapheresis. The prednisolone and plasmapheresis were gradually tapered and stopped over 3 months. There was no relapse over the proceeding 8 months of follow-up.
Discussion
This case describes an unusual presentation of IgA nephropathy with pulmonary hemorrhage. As pulmonary hemorrhage is an unusual presentation of IgA nephropathy and occurred independently of renal manifestations in our patient, this case presented a diagnostic challenge when pulmonary symptoms suddenly developed. There was no evidence for infection, volume overload or bleeding tendency and bronchoscopy suggested diffuse-alveolar hemorrhage. At the time of renal biopsy, we considered the possibility of superimposed glomerulonephritis including pauci-immune vasculitis, Goodpasture syndrome, or lupus nephritis. However, the biopsy result was compatible with IgA nephropathy and autoantibodies were all negative. As there was no purpura or joint symptoms, Henoch-Schönlein purpura was also excluded in this case. Thus, with the possibility of pulmonary renal syndrome in IgA nephropathy, plasmapheresis was performed in spite of there being no definite evidence for vasculitis. There was a similar report that pulmonary capillaritis in IgA nephropathy occurred after the renal pathology progressed to ESRF3). Pulmonary hemorrhage in IgA nephropathy is a rare but severe manifestation with high mortality. Thus, the clinician should consider the possibility of pulmonary renal syndrome and start treatment even in exceptional situations such as that presented herein.
There are two pediatric cases of pulmonary hemorrhage associated IgA nephropathy. Yum et al.7) reported a 17-year-old boy with recurrent respiratory illness and renal insufficiency diagnosed as IgA nephropathy. Treatment with prednisone resulted in normal renal function, but interstitial fibrosis on chest x-ray persisted7). The other case was in a 14-year-old male who presented with ESRF and pulmonary hemorrhage8). He was found to have IgA nephropathy by renal biopsy and pulmonary capillaritis by open lung biopsy8). Pulmonary hemorrhage was successfully treated with steroids and cyclophosphamide8). Our patient is the first pediatric patient with IgA nephropathy and pulmonary hemorrhage who was successfully treated with plasmapheresis.
In the literature, there is no known definitive treatment for pulmonary hemorrhage associated with IgA nephropathy. However, previously reported cases have made use of immunosuppressive therapy in spite of the lack of evidence for this approach23478). Steroids and cyclophosphamide have commonly been used and in limited cases there was recovery after plasmapheresis23478). In ANCA-associated vasculitis, high-dose methylprednisolone and cyclophosphamide are required as induction therapy and although it is known that plasmapheresis reduces progression to ESRF, the benefit in pulmonary hemorrhage treatment is not evident10). In Goodpasture syndrome, corticosteroids and cyclophosphamide are usually used alongside daily plasmapheresis10). In lupus nephritis, intravenous corticosteroids and/or cyclophosphamide are used in pulmonary hemorrhage and plasmapheresis may be useful when other treatments fail10). It is also supposed that plasmapheresis may remove pathogenic molecules from the blood of IgA nephropathy patients. There has been a reported adult case of alveolar hemorrhage in IgA nephropathy treated with plasmapheresis2). A 66-year-old man presented with diffuse alveolar hemorrhage and rapidly progressive glomerulonephritis due to IgA nephropathy, and hemorrhage and renal function improved after high-dose corticosteroid and plasmapheresis2). In our case, plasamapheresis was considered because of the rapid deterioration of renal function before pulmonary symptoms developed. However, plasmapheresis was not carried out at this point because there was no evidence of vasculitis. After he was started on daily plasmapheresis because of life threatening pulmonary hemorrhage, the plasmapheresis was gradually tapered and stopped over 3 months, despite the lack of guidelines for plasmapheresis.
This case suggests that pulmonary hemorrhage may develop independently of renal manifestation and plasmapheresis should be considered as adjunctive therapy to immunosuppressive medication in IgA nephropathy with pulmonary hemorrhage.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.