Clinical experience with 18F-fluorodeoxyglucose positron emission tomography and 123I-metaiodobenzylguanine scintigraphy in pediatric neuroblastoma: complementary roles in follow-up of patients
Article information
Abstract
Purpose
To evaluate the potential utility of 123I-metaiodobenzylguanine (123I-MIBG) scintigraphy and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) for the detection of primary and metastatic lesions in pediatric neuroblastoma (NBL) patients, and to determine whether 18F-FDG PET is as beneficial as 123I-MIBG imaging.
Methods
We selected 8 NBL patients with significant residual mass after operation and who had paired 123I-MIBG and 18F-FDG PET images that were obtained during the follow-up. We retrospectively reviewed the clinical charts and the findings of 45 paired scans.
Results
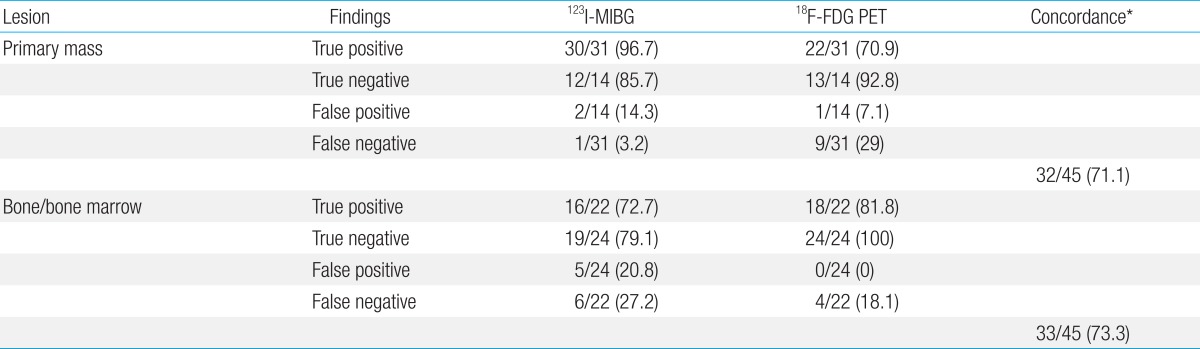
Both scans correlated relatively well with the disease status as determined by standard imaging modalities during follow-up; the overall concordance rates were 32/45 (71.1%) for primary tumor sites and 33/45 (73.3%) for bone-bone marrow (BM) metastatic sites. In detecting primary tumor sites, 123I-MIBG might be superior to 18F-FDG PET. The sensitivity of 123I-MIBG and 18F-FDG PET were 96.7% and 70.9%, respectively, and their specificity were 85.7% and 92.8%, respectively. 18F-FDG PET failed to detect 9 true NBL lesions in 45 follow-up scans (false negative rate, 29%) with positive 123I-MIBG. For bone-BM metastatic sites, the sensitivity of 123I-MIBG and 18F-FDG PET were 72.7% and 81.8%, respectively, and the specificity were 79.1% and 100%, respectively. 123I-MIBG scan showed higher false positivity (20.8%) than 18F-FDG PET (0%).
Conclusion
123I-MIBG is superior for delineating primary tumor sites, and 18F-FDG PET could aid in discriminating inconclusive findings on bony metastatic NBL. Both scans can be complementarily used to clearly determine discrepancies or inconclusive findings on primary or bone-BM metastatic NBL during follow-up.
Introduction
Neuroblastoma (NBL) is the most common extracranial solid tumor in children1). It arises from embryonic neural crest cells derived from the peripheral sympathetic nervous system. Primary tumors can arise anywhere from the pelvis to the neck, and approximately 50% of patients present with distant metastasis to bone, bone marrow (BM), lymph node, and liver2). These clinical characteristics make assessment of the disease status dependent on a multitude of studies.
Functional imaging with the catecholamine analogue 123I-metaiodobenzylguanine (123I-MIBG) has been widely used to assess tumors of the sympathetic nervous system since its development in the early 1980s3). 123I-MIBG scan was well proved its high diagnostic accuracy for NBL assessment in the diagnosis, staging, treatment response and recurrence4). 123I-MIBG scan showed high sensitivity and specificity in identifying metastatic involvement of the cortical bone, BM, and lymph nodes5,6). However, 123I-MIBG still has limitations in the interpretation of the normal distribution pattern in children7), and in the appearance of false-negative results8), which may lead to down-staging9).
18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) has recently emerged as a promising functional imaging modality for various types of adult cancers, and is most frequently investigated in lymphomas, brain tumors and sarcomas. However, no sufficient studies have investigated the use of 18F-FDG PET in NBL. Although 18F-FDG PET showed tumor avidity for revealing NBL in both soft tissue and skeletons, providing a good correlation with the disease status10), questions still remain regarding when and in which patients 18F-FDG PET is most useful11,12,13).
The purpose of the present study was to evaluate the potential utility of 123I-MIBG scintigraphy and 18F-FDG PET for the detection of primary and metastatic lesions of NBL, and to decide whether 18F-FDG PET was as beneficial as 123I-MIBG scintigraphy in NBL patients.
Materials and methods
1. Patients
We selected 8 patients with NBL diagnosed and treated at Ewha Womans University Mokdong Hospital between November 2005 and January 2013, who had residual mass due to incomplete tumor removal and who were able to simultaneously had both 123I-MIBG and 18F-FDG PET scans during follow-up. Their clinical charts and imaging findings were retrospectively reviewed with the approval from Institutional Review Board.
All patients underwent staging evaluation in accordance with the International Neuroblastoma Staging Systems (INSS)14), consisting of 123I-MIBG scans, 99mTc-methylenediphosphonate (MDP) bone scans, computed tomography (CT) and/or magnetic resonance imaging (MRI), urine vanillymandelic acid, urine homovanillic acid, neuron specific enolase and BM examination.
The patients were treated with neo-adjuvant chemotherapy, surgery, 2 or 3 cycles of postoperative chemotherapy, high-dose chemotherapy and autologous stem cell transplantation according to our institutional protocol.
The paired 123I-MIBG and 18F-FDG PET scans were obtained during follow-up schedule at designated times, including at diagnosis, after 4 or 5 cycles of neoaduvant chemotherapy, after surgery and after stem cell transplantation, to define accurate assessment of disease status according to treatment in the patients with residual mass during follow-up. The CT and/or MRI and 99mTc-MDP bone scan were also performed simultaneously. All imaging modalities were obtained within 14 days of each other after informed consent was obtained.
2. Scan acquisitions
123I-MIBG scans were performed with the thyroid protection by ingestion of potassium iodide solution (10 mg/mL) (Lugol's solution) to block 123I-MIBG uptake in the thyroid glands. The dosages of 123I-MIBG were measured according to the body weight (0.2 mCi/kg). Multiple spot imagings of the entire body were acquired at 4 and 20 hours after the injection of 123I-MIBG.
18F-FDG PET scans were preceded by a 6-hour period of fasting with water intake allowed. The dosage of 18F-FDG was 5.18-7.12 MBq/kg with a maximum dosage of 440 MBq (10 mCi). Whole-body scans were performed using a PET/CT scanner 60 minutes after the infusion of 18F-FDG. At first, CT scan was obtained, and transmission images were obtained after the emission scan. The images were reconstructed using filtered back-projection and iterative algorithm, and segmented attenuation and scatter correction was performed. A standardized uptake value (SUV), which is a quantitative measurement of tumor uptake of the 18F-FDG adjusted for injected dose and body weight, was calculated for each lesion, and the maximum SUV of each lesion was determined from the image.
3. Image analysis
All 123I-MIBG and 18F-FDG PET scan images were reviewed by well experienced nuclear medicine physician blinded to the clinical data of the patients. Images for both 123I-MIBG and 18F-FDG PET scans were analyzed by direct comparison on a patient basis as positive or negative, and recorded separately for the primary mass and bone/BM compartment instead of semiquantitative scoring systems for NBL15). On the 18F-FDG PET scan, lesion was interpreted as positive lesion if lesion have focally increased 18F-FDG uptake exceeding normal background uptake.
During the routine follow-up, patients were followed by diagnostic CT or MRI of the abdomen or thorax every 6-12 months, and these morphological imaging were compared with the functional imaging for verification of the lesions. For the bone-BM lesion, BM examination by bilateral aspiration and biopsy at diagnosis and follow-up examinations and 99mTc-MDP bone scans were performed in each case. For the verification of lesions, histopathological finding, follow-up evaluation, and clinical response were used because histopathological verification of all metastases is impossible. A lesion was classified as either "false-positive" or "true-negative" if it disappeared without any therapy during the follow-up period, or if lesion did not have viable tumor by histological verification. A lesion was classified as either "true-positive" or "false-negative" if it persisted or progressed during follow-up, or showed objective regression with therapy13).
Results
The clinical characteristics of the 8 patients are described in Table 1. There were 3 males and 5 females and their median age at diagnosis was 3 years 6 months (range, 2 years 9 months to 5 years 6 months) and their INSS stages were; stage III in one and stage IV in seven cases. The primary tumor sites were; adrenal gland (n=5), posterior mediastinum (n=2), and organ of zuckerkandi (n=1). The patients had variable sizes of residual mass on the primary site after operation with range from about 1 cm to 6 cm in maximum diameter.
To define clinical significance of the residual tumor at the primary site and metastatic sites, a total of 45 combined 123I-MIBG and 18F-FDG PET scans were obtained from eight patients during the follow-up. The frequency of paired scan per patient were; six in patient 1, six in patient 2, seven in patient 3, four in patient 4, six in patient 5, four in patient 6, six in patient 7, and six in patient 8.
1. Detection of posttherapeutic residual tumor at the primary site
The patients had evidence of a residual primary tumor in the postoperative CT scans showing about an 1- to 6-cm range. All residual lesions were examined by CT, 18F-FDG PET, 123I-MIBG scans and 99mTc-MDP bone scans to assess their viability.
A total 30 out of 45 studies were positive on 123I-MIBG images, whereas 37 out of 45 scans were positive on 18F-FDG PET images. The sensitivity of both modalities for NBL detection was 96.7% in 123I-MIBG scan and 70.9% in 18F-FDG PET scan.
On the primary tumor sites, 18F-FDG PET and 123I-MIBG scans were concordant for the positive or negative avidity in 32 of 45 scans (71.1%) at diagnosis and during follow-up (Table 2). The concordance between the both scans was observed 5 of 8 patients (62.5%).
123I-MIBG scintigraphy and 18F-FDG PET imaging findings of 45 paired scans at the primary tumor and metastatic bone-bone marrow sites in 8 patients
The 18F-FDG PET scans missed 9 studies out of 31 images (29%), and the 123I-MIBG scan missed 1 study out of 31 images (3.2%) for detection of NBL in primary tumor sites (Table 2). The patient 3, who had a large residual abdominal mass (lesion diameter 4.5 cm×5.5 cm) was observed with positive uptake for 123I-MIBG but a negative result in the 18F-FDG PET scan (Fig. 1). These false-negative findings were repeatedly observed in 2 out of 7 follow-up scans. Finally, the negative avidity on the 18F-FDG PET scan was false negative finding, which was confirmed as viable tumor by biopsy.
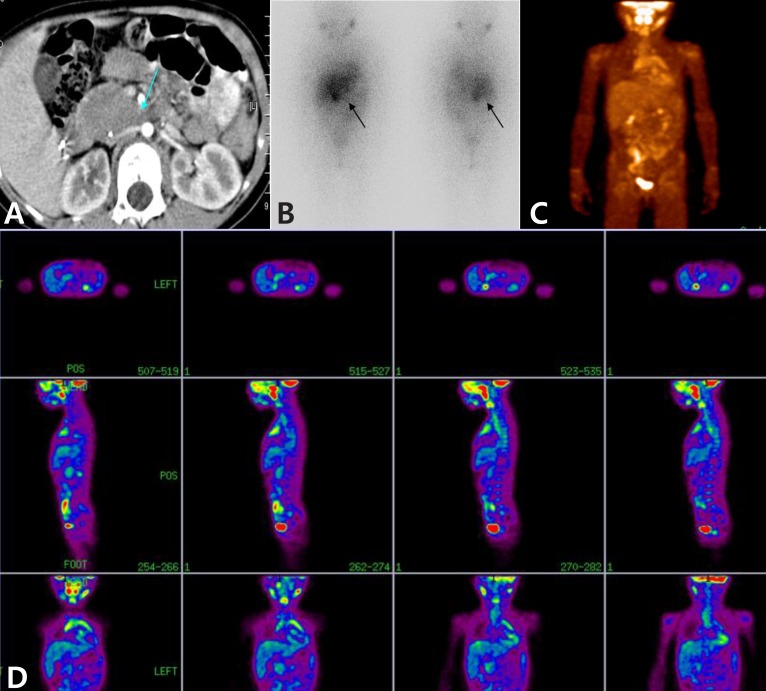
A 6-year-old female patient with neuroblastoma in the adrenal gland and the false-negative finding on 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET). She had a large residual abdominal mass (lesion diameter, approximately 4.5 cm×5.5 cm) on computed tomography after autologous stem cell transplantation (arrow) (A). After 7 months, 123I-metaiodobenzylguanine (123I-MIBG) scintigraphy images show a lesion in the right adrenal region (arrows) (B), 18F-FDG PET images do not show any positive uptake (C, D).
The false positive findings were comparable between 123I-MIBG and 18F-FDG PET images, which were shown in 2 out of 14 scans (14.3%) on 123I-MIBG and in 1 out of 14 scans (7.1%) at the primary tumor sites at diagnosis and during follow-up (Table 2, Fig. 2). These results implicated 18F-FDG PET scan was worse in depicting disease in the soft-tissue compartment, and the 123I-MIBG scan might be superior to 18F-FDG PET for detecting a region of the primary tumor.
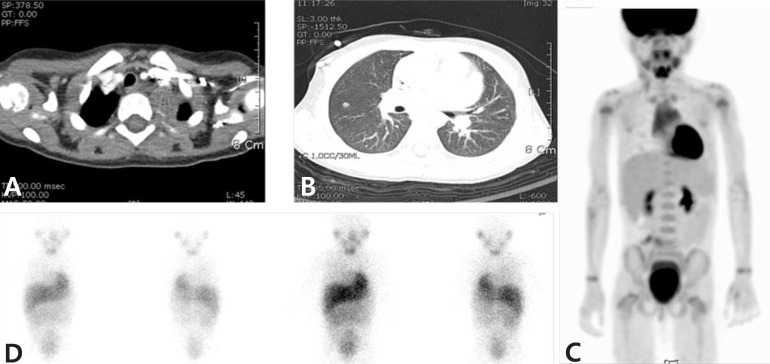
A 3-year-old female patient with stage IV neuroblastoma in the posterior mediastinum and the false-positive finding on 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) scan. She had a residual primary mass (size, approximately 3 cm×4cm) in the posterior mediastinum after operation (A). During follow-up, we found suspicious metastatic nodules in both the upper and right middle lung on computed tomography (B). These nodules showed positive result in 18F-FDG PET (C), but were negative in 123I-metaiodobenzylguanine scans (D). Finally, the nodules disappeared and no recurrence was observed during the follow-up.
The 123I-MIBG scintigraphy, showed a false negative finding in 1 of 31 scans (3.2%), and false positive findings in 2 of 14 scans (14.3%) at the primary tumor sites at diagnosis and during follow-up (Table 2). The patient 7, had a residual mass (adrenal gland, diameter less than 1 cm) after operation. The residual mass twice showed negative findings on 123I-MIBG scans, but positive on 18F-FDG PET scans. 18F-FDG PET scan can be useful identify an active viable tumor in an 123I-MIBG negative lesion (Fig. 3). The patient 2 who had a residual mass (adrenal gland, diameter 3 cm×4 cm) had been monitored regularly, and showed positive uptake in an 123I-MIBG scan but negative in 18F-FDG PET at 10 months after treatment completion. Both scans repeated nine months afterwards, and the positive avidity on the 123I-MIBG scan was converted to negative imaging without any treatment with good clinical status.

A 5-year-old-male patient with stage 4 neuroblastoma in the adrenal gland and the false-negative finding on 123I-metaiodobenzylguanine (123I-MIBG) scintigraphy. He had a residual mass in the adrenal gland, with diameter less than 1 cm (arrow) (A). The residual mass showed negative finding on 123I-MIBG scintigraphy (B), but positive on 18F-fluorodeoxyglucose positron emission tomography (arrow) (C).
These results indicated that the 123I-MIBG scan might be superior to 18F-FDG PET for detecting a region of the primary tumor.
2. Detection of bone-BM involvement
A total 21 123I-MIBG images were positive in 45 scans, whereas 18F-FDG PET images were positive in 18 of 45 scans. The sensitivity of both modalities for NBL detection was 72.7% in 123I-MIBG scan and 81.8% in 18F-FDG PET scan.
In the evaluation of bone-BM metastatic sites, 18F-FDG PET and 123I-MIBG scans were concordant for the positive or negative avidity in 33 of 45 scans (73.3%) at the diagnosis and during the follow-ups (Table 2). The concordance between the both scans was observed 6 of 8 patients (75%). Nevertheless, both 18F-FDG PET and 123I-MIBG scans missed metastatic bone-BM lesions in 3 studies although the 99mTc-MDP bone scan showed disseminated involvement of multiple skeletal uptake (Fig. 4).
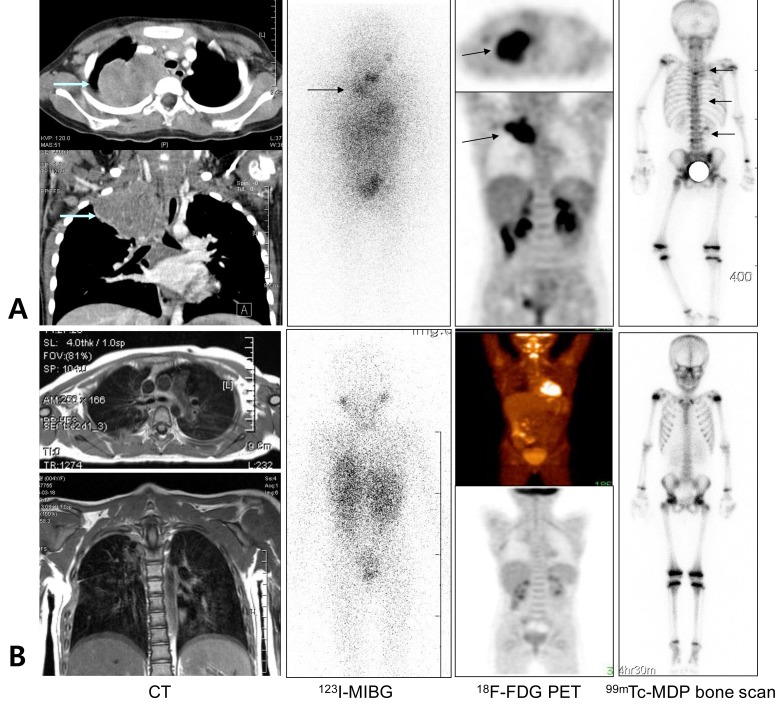
A 5-year-old female patient with stage IV neuroblastoma in the right posterior mediastinum. (A) 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) and 123I-metaiodobenzylguanine (123I-MIBG) scans showed similar results, in which the neck and posterior mediastinal primary mass are well-delineated with 18F-FDG PET and 123I-MIBG scans at diagnosis (arrows). However, a false-negative findings were obtained with regard to bone involvement. (B) Ten months after undergoing high-dose chemotherapy and autologous stem cell transplantation, 18F-FDG PET and 123I-MIBG scans showed similar negative results with regard to the previous neck and posterior mediastinal primary mass.
Both modalities showed overall equivalence in detecting bone or BM metastasis; 18F-FDG PET scans missed 4 studies (8.9%) of 2 patients (Fig. 4) and 123I-MIBG scans missed 6 studies (13.3%) of 3 patients. However, 123I-MIBG scan showed higher presentation of false positive findings than 18F-FDG PET scan. There were false positive findings in 5 out of 24 scans (20.8%) by 123I-MIBG scan, but there were no false positive findings in 18F-FDG PET scan in bone-BM sites during the follow-ups of NBL patients.
These results implicated that 18F-FDG PET might be better for detecting bone or BM metastasis than 123I-MIBG scan. The bone scan did not precisely detect bone lesions, and it missed active bony lesion in 4 studies (8.8%) of patient 5. Data in all patients are summarized on Table 3.
Discussion
In this retrospective study, we investigated whether 18F-FDG PET was useful as much as 123I-MIBG scintigraphy in detecting the primary and metastatic lesions during the follow-up of high-risk NBL patients. In our 8 patients with high risk NBL, 45 studies were performed. And they showed that both modalities are relatively well-correlated with the disease status as determined by standard imaging modalities during follow-ups; overall concordance rates were 32/45 (71.1%) on primary tumor sites and 33/45 (73.3%) at bone-BM metastatic sites.
Many authors have been investigating to assess the potential utility of 18F-FDG PET and 123I-MIBG scans in children with NBL. So far, there are no conclusive reports to support which modality is more useful for monitoring the primary and metastatic lesions during follow-up. 123I-MIBG scintigraphy has been widely used in clinical settings for the past 25 years in pediatric oncology providing high sensitivity and specificity in NBL. Nevertheless, it still has limitations in interpretation of unusual appearance of normal physiologic uptake such as upper thoracic and gastric region in children7), and leading to false-negative results8,9). Moreover, there are some difficulties in detecting lesions during the follow-ups of NBL since 123I-MIBG positivity on initial diagnosis may reverse negative avidity when the disease relapses16). Although 18F-FDG PET is increasingly used to overcome these limitations, 18F-FDG uptake into nontumor sites can cause potential false-positive or false-negative results. Also, there is no evidence to support the use of 18F-FDG PET in the initial diagnosis.
In our study, we separately assessed avidity between primary and metastatic bony lesions. In detecting the primary tumor sites, 123I-MIBG scans might be superior to 18F-FDG PET, and the sensitivity values of 123I-MIBG and 18F-FDG PET were 96.7% and 70.9%, respectively, and their specificity values were 85.7% and 92.8%, respectively. Although 18F-FDG PET was able to reveal the metabolic states of the primary or residual NBL as much as 123I-MIBG, 18F-FDG PET missed 9 true NBL lesions in 45 follow-up scans (29% false negative rate) with a positive 123I-MIBG, in which histopathologically viable tumor activity was proven (patient 3). 18F-FDG PET did not show any advantage over 123I-MIBG. Our results accord with the several of the recent studies11,17). Shulkin et al.11) reported that 123I-MIBG scan was superior to 18F-FDG PET, which showed lower tumor-to-nontumor uptake ratio and physiological 18F-FDG accumulation, for NBL tumor delineation. Sharp et al.17) reported that 123I-MIBG scan was superior to 18F-FDG PET in 40 patients with stage IV NBL. 123I-MIBG was more sensitive for bone lesions than 18F-FDG PET in patients of the New Approaches to Neuroblastoma Therapy (NANT) trial18).
18F-FDG PET might be better in detection for bone or BM metastases during follow-up than 123I-MIBG scan. The sensitivity values of 123I-MIBG and 18F-FDG PET were 72.7% and 81.8%, respectively, and their specificity values were 79.1% and 100%, respectively. 123I-MIBG scans showed a higher prevalence of false positivity (20.8%) than 18F-FDG PET (0%). 18F-FDG PET provided important information for the patient with false positive lesions on 123I-MIBG at the major decision points during follow-up (patient 2). Therefore, 18F-FDG PET may be useful to clearly discriminate discrepancies, or inconclusive findings on the bone/BM metastatic NBL during follow-up.
The role of 18F-FDG PET for detecting bone or BM metastases is controversial. Kushner et al.12) reviewed 92 18F-FDG PET scans obtained from 51 patients with NBL, and reported that 18F-FDG PET is better than 123I-MIBG for detection of both soft tissue and extracranial osteomedullary lesions12). Colavolpe et al.16) reported that 18F-FDG PET scans are helpful in detecting the multiple hypermetabolic osteo-medullary metastasis in the patients with negative 123I-MIBG scintigraphy. In contrast, several studies reported 123I-MIBG scan is superior to 18F-FDG PET in the assessment of disease extent in high-risk NBL18,19). 123I-MIBG was more sensitive for bone lesions than 18F-FDG PET in patients of the NANT trial18). Consequently, it still remains difficult to predict the clinical roles and accuracy of 18F-FDG PET scans in NBL.
123I-MIBG is known to be highly sensitive and specific for identifying metastatic involvement of cortical bone and BM, and 18F-FDG PET was better for identifying soft tissue lesions. However, our study showed conflicting results for identifying individual lesion in NBL. The discrepancy between the two modalities for detecting individual tumor lesions may be due to small sample size, preselected high-risk study populations, and variability of tumor tracer uptake in different radiopharmaceutical. Actually, mechanism of tracer uptake is different between 123I-MIBG and 18F-FDG. MIBG is an analog of the adrenergic norepinephrine neurotransmitter, which is taken by the adrenergic origin of NBL and pheochromocytoma using the type 1 cathecolamine uptake mechanism for transport into tumor cells17). Uptake of 123I-MIBG was highly variable in vivo, and probably influenced by multiple factors such as degree of differentiation in neuroendocrine tumor, interfering medication, and change in the metabolism of the malignant cells due to altered biology13,20). 18F-FDG, similar to glucose, is transported to within the cells via the GULT (glucose transporters) and intracellular phosphorylated by hexokinase into glucose-6-phosphate and 18F-FDG-6-phosphate. 18F-FDG-6-phosphate entrapped, and steadily accumulates in metabolically active cells with different uptake mechanism with 123I-MIBG21). Although most NBL and associated metastases have been shown to have good avidity for FDG, 18F-FDG PET have shown variable results during/after treatment due to its lower tumor-to-nontumor uptake ratio and FDG uptake in nontumor sites4,22). 18F-FDG PET also show false positive results in osteo-medullary assessment due to diffuse BM uptake after treatment (hyperactivity) with growth factors or radio-chemotherapy (rebound after treatment).
Another possibility for discrepant result is spontaneous regression or cellular differentiation of NBL tumor cells leading to false negative activity in 123I-MIBG scan due to modification in the uptake mechanism, but we did not analyzed as a contributory factor in this study.
Our study showed both 123I-MIBG and 18F-FDG PET scans complement each other which is comparable to the recent study by Melzer et al.13). In their study, they showed the sensitivity values of 123I-MIBG scintigraphy and 18F-FDG PET to be 50% and 78%, respectively, and their specificity values to be 85.7% and 92%, respectively. They recommended 18F-FDG PET as an additional modality when the results were discrepant or inconclusive with 123I-MIBG, 18F-FDG PET, and morphological imaging during the follow-up.
The limitations of this study is the small number of cases, which could have an influence in determining concordance or discordance rate, and the sensitivity or specificity. We recalculated the sensitivity and specificity from each patient to evaluate their effect. We finally confirmed that there was very little difference in values. Our study population to selective patients in the high risk NBL, who had residual tumors, and who were available for paired scans. The sensitivity and specificity of 123I-MIBG scintigraphy is not applicable to the general population of NBL patients. This makes it difficult to compare our study results with the studies already published in the literature.
In conclusion, the functional studies, such as 123I-MIBG and 18F-FDG PET scans, surely are valuable in distinguishing the residual active disease from residual operative scars. However, 18F-FDG PET cannot replace 123I-MIBG or bone scans in the evaluation for primary or metastatic NBL disease during the follow-up, because they show relatively higher false-negative results. Both scans have complementary roles to clearly discriminate discrepancies, or inconclusive findings on the primary or bone-BM metastatic NBL during the follow-up. Further studies in larger sample size will be needed.
Notes
No potential conflict of interest relevant to this article was reported.