Associations of matrix metalloproteinase (MMP)-8, MMP-9, and their inhibitor, tissue inhibitor of metalloproteinase-1, with obesity-related biomarkers in apparently healthy adolescent boys
Article information
Abstract
Purpose
Matrix metalloproteinases (MMPs) have been implicated in atherosclerosis, and therefore, are considered risk factors for metabolic dysfunction in adults. However, there is little data on circulating levels of MMPs and tissue inhibitors of MMPs (TIMPs) with regard to obesity-related biomarkers in the general adolescent population. In the present study, we determined the associations of MMP-8, MMP-9, and TIMP-1 levels and MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios with obesity-related biomarkers in apparently healthy adolescent boys.
Methods
We measured MMP and TIMP concentrations in plasma samples using the enzyme-linked immunosorbent assay and analyzed their associations with obesity-related biomarkers, such as liver enzymes and lipid profiles, in a sample of 91 Korean boys aged 13-14 years who participated in a general health check-up.
Results
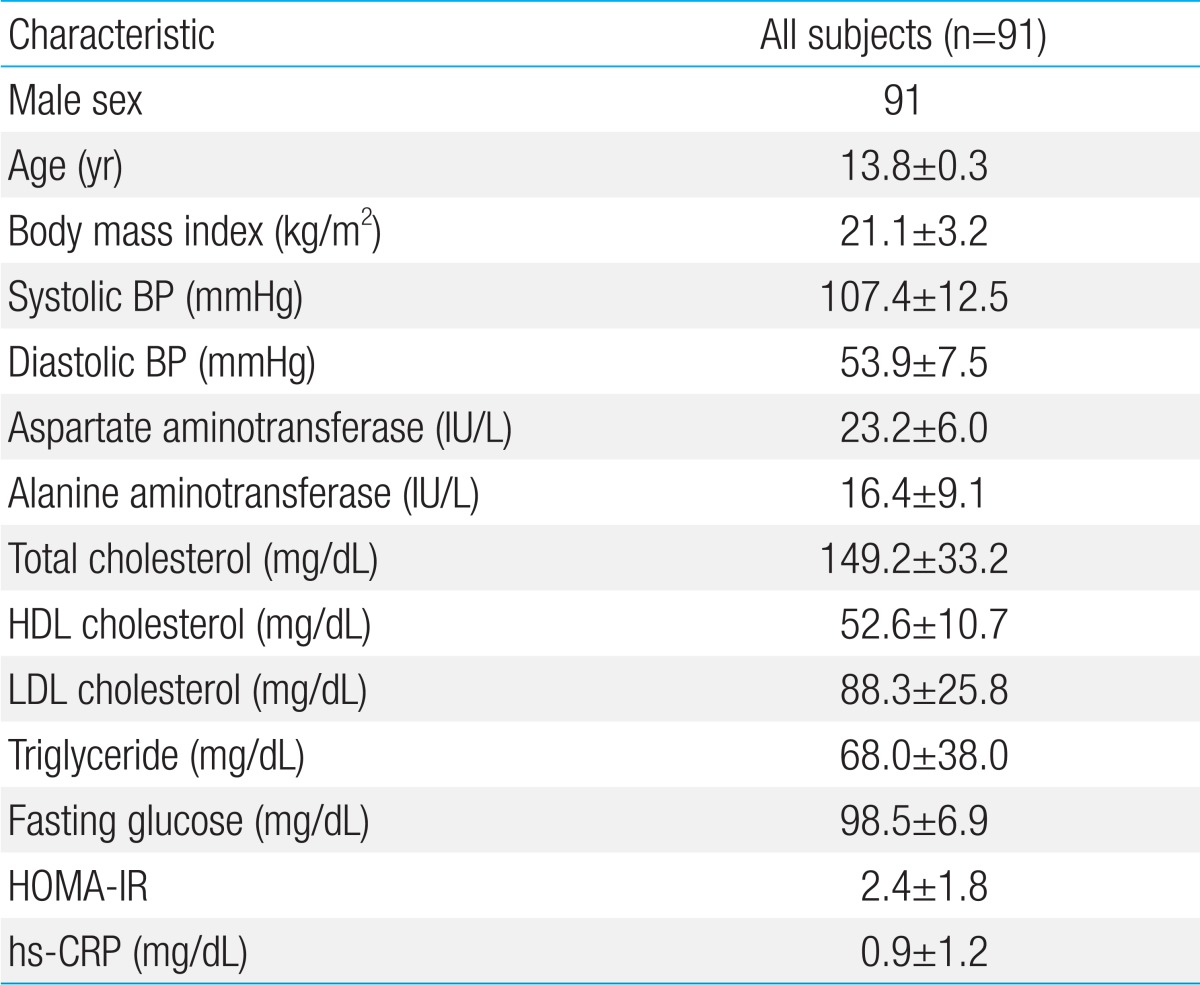
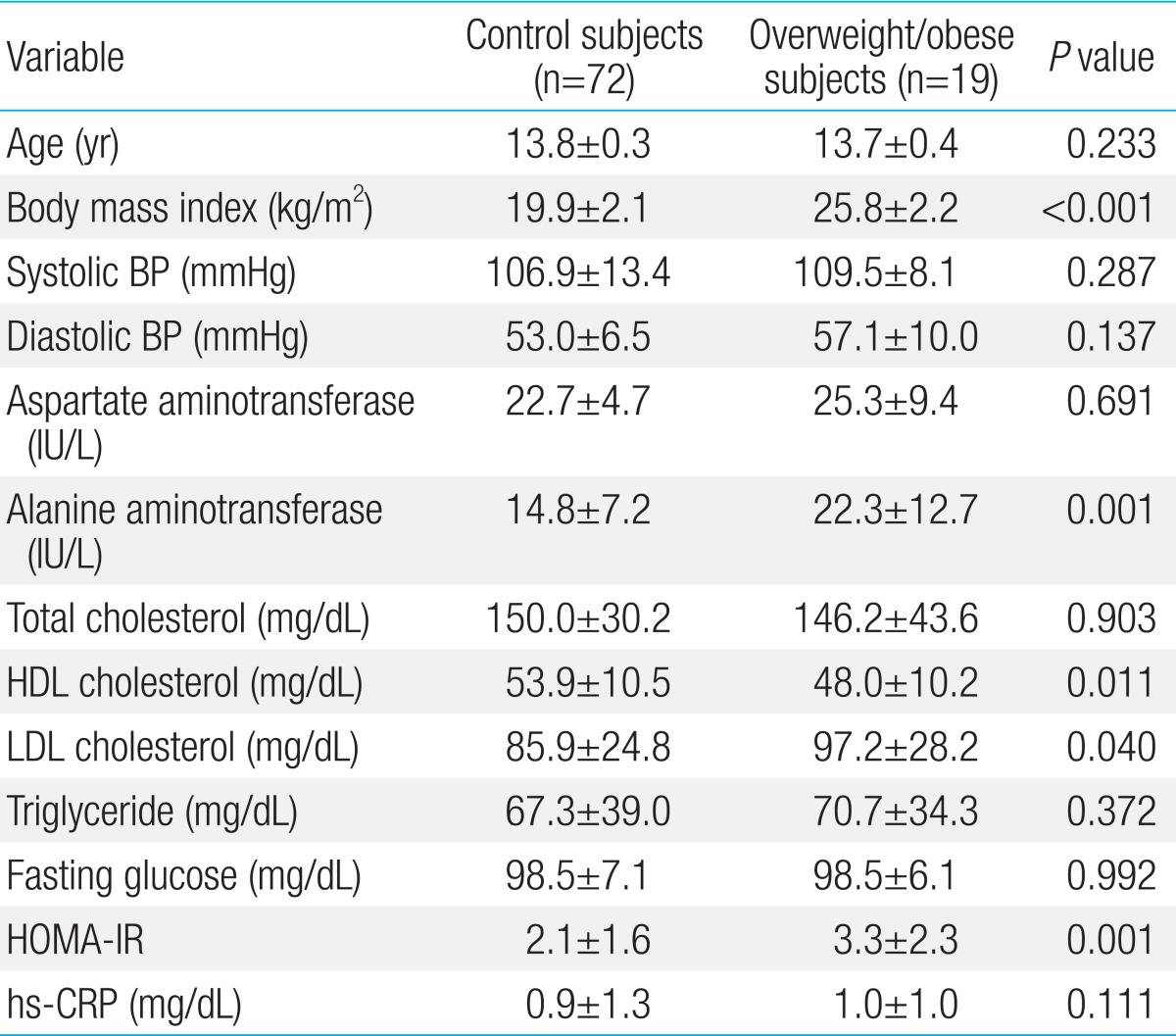
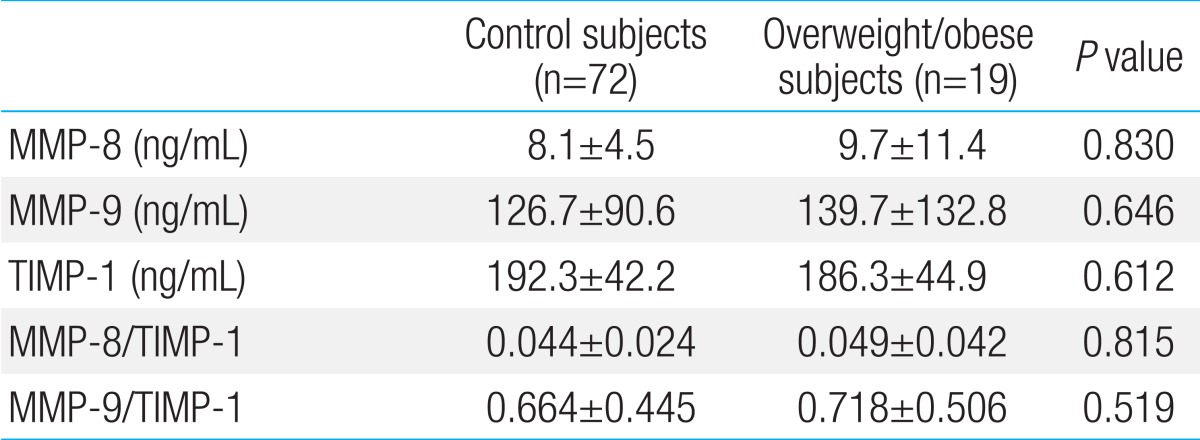
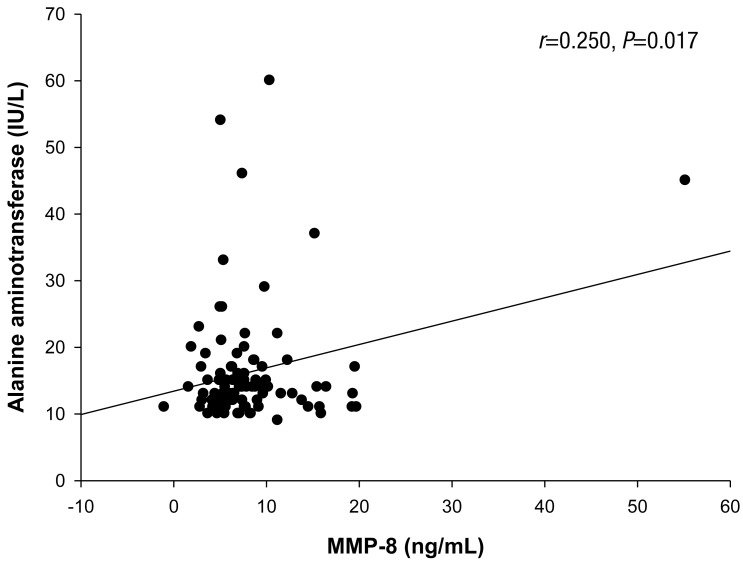
The mean age of the boys was 13.8±0.3 years; 72 boys were normal weight and 19 were overweight/obese. The Pearson correlation coefficients revealed a significant correlation between MMP-8 and aspartate aminotransferase (r=0.217, P=0.039) and alanine aminotransferase (r=0.250, P=0.017) and between TIMP-1 and aspartate aminotransferase (r=0.267, P=0.011). In a multivariate linear regression analysis, serum alanine aminotransferase was positively associated with the MMP-8 level. There were no significant differences in the MMP-8, MMP-9, and TIMP-1 levels or MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios between control and overweight/obese subjects.
Conclusion
We found a significant association between the MMP-8 level and alanine aminotransferase in the apparently healthy adolescent boys. These findings indicate that there may be a pathophysiological mechanism underlying the relationship between MMP-8 and liver enzymes in young adolescents.
Introduction
Obesity has become a large public health concern and is now occurring at younger ages worldwide1). Compelling evidence suggests that childhood overweight and obesity often persists into adulthood and is associated with increased morbidity and mortality in adulthood2). This metabolic disorder increases the risk of cardiovascular diseases, ultimately causing premature death3). Studies have suggested that adipocytes not only store fat, but are also an active endocrine and paracrine organ that releases various bioactive mediators that influence body weight homeostasis, insulin resistance, circulating lipid levels, arterial blood pressure (BP), and coagulation4).
A growing body of research into the role of adipocytes also suggests that they play an important role in inflammatory mechanisms, leading to the development of atherosclerosis4,5). Atherosclerosis is characterized by vascular remodeling and accumulation of lipids and fibrous elements in the large arteries6). Several members of the matrix metalloproteinase (MMP) family and their endogenous inhibitors, the tissue inhibitors of MMPs (TIMPs), have been suggested to be primary mediators of vascular remodeling7,8). Therefore, a critical equilibrium between MMPs and TIMPs must exist to retain cardiovascular integrity and to prevent the development of cardiovascular diseases7,8). Laboratory and clinical studies have reported that modified expression or activity of MMPs, TIMPs, or both is essential in the pathophysiology of various cardiovascular diseases8,9,10,11) and obesity12,13,14).
Despite mounting evidence that MMPs and TIMPs play a role in the pathophysiology of cardiovascular diseases8,9,10,11) and obesity12,13,14), no previous work has studied the relationships of circulating concentrations of MMP-8, MMP-9, and TIMP-1 levels and their ratios with obesity-related biomarkers in the general population of adolescents. Understanding these relationships could help determine if atherosclerosis develops early in childhood and gradually accelerates during adolescence in obese children, as has been previously suggested.
Therefore, the aim of the present study was to determine the relationships of MMP-8, MMP-9, and TIMP-1 levels and the MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios with anthropometric measures and obesity-related biomarkers, such as liver enzymes and lipid profiles, in apparently healthy adolescent boys.
Materials and methods
1. Study subjects
One hundred twelve adolescent boys aged 13 to 14 years underwent Student Health Examinations at their school in Seoul, Korea in May 2012. Subjects meeting any of the following criteria were excluded (n=21): any missing covariate information; a medication history of steroids, insulin, glucose regulators, or antihypertensive drugs; subjects who refused the test; subjects who had not fasted for at least 12 hours before blood sampling. After these exclusions, 91 subjects (all boys) were included in our analysis. Health examinations were performed by a single physician according to a standardized procedure. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with an automatic height-weight scale while subjects wore light indoor clothing and no shoes. BMI was calculated as weight (kg)/height (m2). The BMI percentiles for age and sex were determined according to the 2007 Korea Growth Charts in order to determine normal-weight, overweight, and obesity15). The subjects were subdivided into two groups according to their BMI value: the normal subgroup (BMI<85th percentile) and the overweight/obese subgroup (BMI≥85th percentile). A certified technician measured BP a maximum of three times on the right arm in seated subjects after a 5-minute rest using an automatic BP recorder. The institutional review board approved the study, and written informed consent was obtained from the subjects or their guardians.
2. Laboratory analyses for obesity-related biomarkers
Following a 12-hour overnight fast, blood samples were obtained from the antecubital vein of each subject by venipuncture and were immediately centrifuged, aliquoted, and frozen at -20℃. The frozen serum and plasma samples were stored at -80℃ until analysis. Fasting plasma glucose (FPG), total cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-C) levels were measured by enzymatic methods using a Hitachi 7600-110 automated chemistry analyzer (Hitachi, Tokyo, Japan). Levels of low-density lipoprotein cholesterol (LDL-C) were calculated using the following formula: LDL-C=total cholesterol-HDL-C-(triglyceride/5). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured. Fasting insulin levels were measured by an electroluminescence immunoassay (Roche, Indianapolis, IN, USA). Insulin resistance was estimated using the homeostatic model assessment of insulin resistance (HOMA-IR) and calculated using the following formula: HOMA-IR=[fasting insulin (µIU/mL)×fasting glucose (mg/dL)/18]/22.516).
3. Plasma MMPs and TIMPs measurement
Concentrations of MMP-8, MMP-9, and TIMP-1 were measured in plasma using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA)13,17) according to the manufacturer's instructions.
4. Statistical analysis
All continuous variables are presented as mean with standard deviation or are shown as scatter plots. The Mann-Whitney U test or the Kruskal-Wallis test was used to examine statistical differences between groups. The Pearson correlation analyses were used to calculate the correlation between plasma MMP or TIMP levels and anthropometric measures and obesity-related biomarkers. To examine independent correlates of plasma MMP-8 levels, a multivariate linear regression analysis was conducted with the MMP-8 level as the dependent variable. All analyses were conducted by using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). All statistical tests were two-sided, and P<0.05 represented statistical significance.
Results
1. Population characteristics
The demographic and clinical characteristics of the study population are presented in Table 1. All subjects were boys, and the mean age was 13.8±0.3 years. The mean clinical and laboratory values were within the normal ranges. Seventy-two subjects were normal-weight and nineteen subjects were overweight (16 subjects) or obese (3 subjects).
2. Comparison of clinical and laboratory parameters between control and overweight/obese subjects
Table 2 compares the clinical and laboratory parameters for control (n=72) and overweight/obese subjects (n=19). BMI, ALT, LDL-cholesterol, and HOMA-IR were significantly higher and HDL-C was significantly lower in the overweight/obese subjects. There were no significant differences in age, systolic and diastolic BP, AST, total cholesterol, triglyceride, fasting glucose, or high sensitivity C-reactive protein between groups.
3. Comparison of MMPs, TIMPs, and their ratios between control and overweight/obese subjects
Table 3 compares MMPs, TIMPs, and their ratios for control and overweight/obese subjects. There were no significant differences in MMP or TIMP concentrations or their ratios between groups.
4. Associations of MMP-8, MMP-9, and TIMP-1 levels, and MMP-8/TIMP-1, and MMP-9/TIMP-1 ratios with anthropometric measures and obesity-related biomarkers
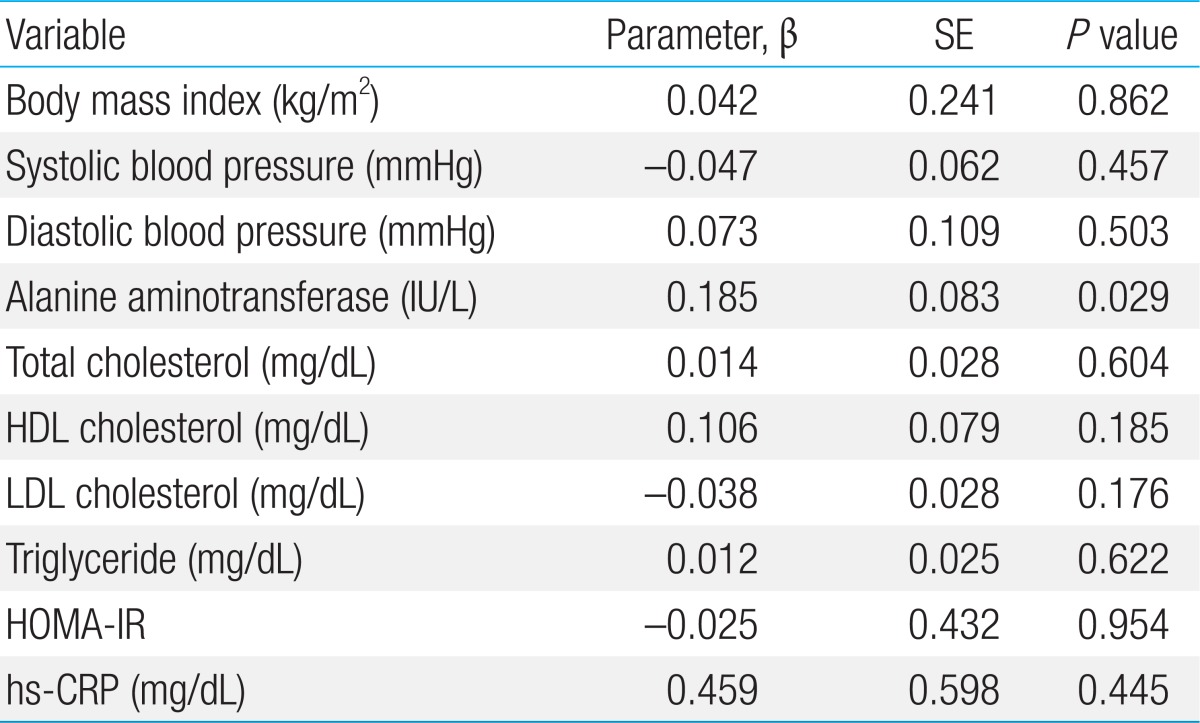
The Pearson correlation results between MMP-8, MMP-9, and TIMP-1 levels, and the MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios and various parameters are listed in Table 4. The MMP-8 level was significantly correlated with AST (r=0.217, P=0.039) and ALT (r=0.250, P=0.017) (Figs. 1, 2; Table 4). The TIMP-1 level was significantly correlated with AST (r=0.267, P=0.011) (Fig. 3, Table 4). There were no significant correlations between MMP-8, MMP-9, and TIMP-1 levels, or their ratios and the other clinical parameters. In the multivariate linear regression, ALT was positively associated with MMP-8 levels (Table 5). There were no significant differences between MMP-8, MMP-9, and TIMP-1 levels, or MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios across serum AST and ALT quartiles (data not shown).
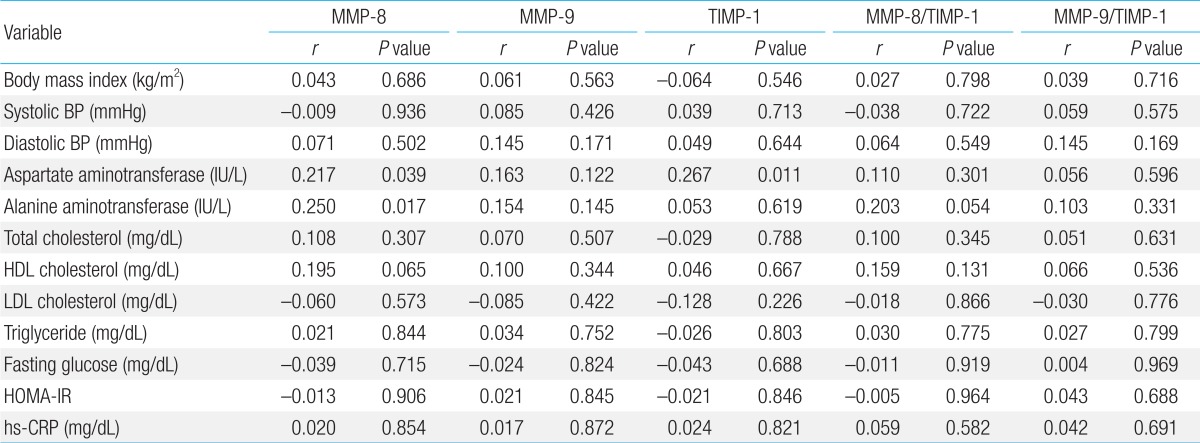
Correlations of MMP-8, MMP-9, TIMP-1, and the MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios with various parameters

The relationship between tissue inhibitor of metalloproteinase (TIMP)-1 and aspartate aminotransferase.
Discussion
The aim of the present study was to determine the relationships of MMP-8, MMP-9, and TIMP-1 levels, and the MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios with anthropometric measures and obesity-related biomarkers, such as liver enzymes and lipid profiles, in apparently healthy adolescent boys. The multivariate linear regression analysis revealed a significant positive association between MMP-8 and ALT. To the best of our knowledge, this is the first study to determine a significant positive association between MMP-8 and ALT in the general population of adolescent boys.
The prevalence of overweight, obesity, and metabolic syndrome is increasing in Korea18) and around the world1). Despite intense research, the pathogenesis and pathophysiology of these complex diseases are not well understood. In recent years, studies have shown that obese children and adolescents have higher circulating MMP-8 concentrations, lower TIMP-1 concentrations, and higher MMP-8/TIMP-1 ratios than controls, suggesting that MMP-8 may be involved in collagen breakdown, thereby increasing vulnerability to atherosclerotic plaques13). In contrast, the present study found no difference in MMP-8, MMP-9, or TIMP-1 levels between control and overweight/obese subjects. This discrepancy may be due to differences in ethnicity, population, or classification of groups.
Emerging evidence has shown that the liver metabolism plays a critical role in the pathogenesis of metabolic syndrome19) and insulin resistance19,20). Nonalcoholic fatty liver disease (NAFLD) is the accumulation of large droplets of triglycerides in liver cells without a history of chronic alcohol consumption and, hence, has been regarded as the hepatic manifestation of overweight21), obesity22), and metabolic syndrome22,23,24). Although liver biopsy is the gold standard for identifying NAFLD, serum biomarkers, notably ALT level, have been shown to be sensitive in diagnosing NAFLD. These biomarkers are also associated with increased risk of overweight, obesity, metabolic syndrome, diabetes mellitus, and cardiovascular disease24,25,26). Recent studies have reported that liver enzymes increase before non-alcoholic fatty liver develops, suggesting that liver enzymes may be a marker of NAFLD19,22,23,24,27,28). Although we did not perform a liver biopsy study, we found that the serum MMP-8 level was positively associated with the ALT level using the multivariate linear regression model.
Why MMP-8, also known as neutrophil collagenase or collagenase-2, is positively correlated with liver enzymes remains unclear. One possible explanation for the association between MMP-8 and ALT is that MMP-8 produced by neutrophils and endothelial cells may be involved in collagen breakdown, leading to increased vulnerability to atherosclerotic plaques11,29). The MMP-8 level has been shown to be positively associated with subclinical and clinical atherosclerosis11,30,31); thus, the MMP-8 concentration has been proposed as a marker of atherosclerosis. Atherosclerosis begins early in life32) and gradually accelerates throughout adolescence in children with risk factors33). A cross-sectional study of 830 healthy adults with normal ALT levels showed that 48.4% of men and 36.7% of women had liver steatosis as determined by ultrasonography34). In women, the carotid intima-media thickness increased significantly with increasing quartiles of ALT levels34). These results suggest that increased ALT level, even within the reference range, is closely correlated with atherosclerotic changes in healthy adults34). Although we did not perform ultrasonography, the subjects with elevated liver enzymes in our study may have already had fatty deposits in their vessels, thereby resulting in a positive association between liver enzymes and MMP-8 concentrations. Another possible explanation for the association between MMP-8 and ALT is as follows: the liver has a rich blood supply, as indicated by its dark red or reddish brown color and the blood vessels are lined with endothelial cells, which produce MMP-8 in the presence of fibrinolytic changes. Thus, liver damage due to hepatic metabolic overload may cause blood vessels to become disorganized, inducing endothelial cells that line the vessels to overproduce MMP-835).
The present study has some limitations. First, this study used a cross-sectional design; therefore, the causality of the association between MMP-8 levels and liver enzymes remains uncertain. However, we speculate that, since MMP has fibrinolytic properties, it may have a significant role in liver metabolism, thereby resulting in elevated liver enzymes. Second, we did not measure other liver enzymes, such as γ-glutamyl transferase and alkaline phosphatase, which are often used as diagnostic markers for liver diseases. Finally, we did not perform ultrasonography on subjects with elevated AST and ALT in order to diagnose NAFLD.
In conclusion, we found evidence of significant positive associations between MMP-8 and TIMP-1 levels and liver enzymes in a general population of apparently healthy adolescent boys. Further research with larger sample sizes is warranted to determine if a pathophysiological mechanism is involved in the associations between MMPs and TIMPs and liver enzymes.
Acknowledgment
This study was supported by a 2011 research grant from the Korean Pediatric Society (MSD Award).
Notes
No potential conflict of interest relevant to this article was reported.