Growth hormone treatment and risk of malignancy
Article information
Abstract
Growth hormone (GH) treatment has been increasingly widely used for children with GH deficiencies as the survival rate of pediatric patients with malignancies has increased. Both GH and insulin-like growth factor-I have mitogenic and antiapoptotic activity, prompting concern that GH treatment may be associated with tumor development. In this review, the authors examined the relationship between GH treatment and cancer risk in terms of de novo malignancy, recurrence, and secondary neoplasm. Although the results from numerous studies were not entirely consistent, this review of various clinical and epidemiological studies demonstrated that there is no clear evidence of a causal relationship between GH treatment and tumor development. Nonetheless, a small number of studies reported that childhood cancer survivors who receive GH treatment have a small increased risk of developing de novo cancer and secondary malignant neoplasm. Therefore, regular follow-ups and careful examination for development of cancer should be required in children who receive GH treatment. Continued surveillance for an extended period is essential for monitoring long-term safety.
Introduction
Growth hormone (GH) has been administered to children with GH deficiency since the late 1950s, initially using extracts from human pituitary glands1). Since the 1980s, GH has been produced by recombinant DNA technology and prescribed for a wide variety of disorders. As the use of synthetic human recombinant GH has increased over the past several decades, there has been a concurrent increase in awareness of the potential safety issues accompanying the treatment2). Recently, Carel et al.3) analyzed a French population-based registry of 6,928 children who started GH treatment between 1985 and 1996. The researchers reported that mortality rates increased in children treated with recombinant GH; however, all types of cancer-related mortality did not increase, and most of the increased mortality was due to bone tumors or cerebral hemorrhage. This exemplifies the controversy associated with GH treatment and risk of malignancy.
Survival rates of pediatric patients with malignancies are increasing due to improved treatment modalities, and interest in improved, long-term quality of life is increasing in tandem, including optimizing the final height of a patient with GH deficiency after cancer treatment4). For patients with GH deficiency after completing antitumor therapy, GH is often recommended to increase height velocity and improve quality of life5).
On its own, however, GH has mitogenic activity6). There have been theoretical and clinical concerns that GH may play a part in tumor recurrence7,8). GH treatment may increase an individual's risk of developing cancer, particularly in the case of cancer survivors, by increasing the risk of recurrence or secondary malignancy9). Controversies and debates are currently ongoing regarding GH treatment's possible causal relationship with tumor recurrence.
It is known from in vitro and in vivo studies that GH and insulin-like growth factor-I (IGF-I) have mitogenic and antiapoptotic activity, and it is possible that GH treatment may be associated with tumor development7,8). Higher serum levels of IGF-I may also be associated with increased cancer risk, and concern has been raised regarding its potential role as a carcinogen after GH treatment10). Some epidemiological studies have shown a correlation between high serum levels of IGF-I and occurrence of certain cancers, including carcinomas of the prostate, lung, breast, and colon11,12). GH receptors have been expressed on normal and transformed human white blood cells, and in vitro studies have shown that GH may cause transformation of normal cells and proliferation of leukemic cells9,13). Most of IGF-I's physical effects are mediated through the type 1 IGF receptor, which is overexpressed in many different types of cancers14). Although the results of all studies are not completely consistent, a high concentration of IGF-I may be linked to an increased risk of cancer development15).
As with IGF-I, there is a concern that GH treatment may be associated with tumor development. Although GH treatment has been shown to be generally safe, it is important to investigate the relationship between GH treatment and cancer risk. The authors reviewed clinical and epidemiological studies that examined cancer risk in patients treated with GH with a focus on de novo malignancy, recurrence, and secondary neoplasm.
GH treatment and de novo malignancy
1. Leukemia
A possible correlation between GH therapy and increased risk of leukemia was reported in Japan16); however, the investigators carried out a follow-up analysis of the cohort and could not find a close association between GH therapy and leukemia when subjects with known risk factors for leukemia were excluded17). The United States' National Hormone Pituitary Program found 3 cases of leukemia in 59,736 patient-years of GH treatment between 1963 and 1985. The rate was not significantly higher than the 1.6 cases expected for an age, ethnicity, and sex-matched population18). Genentech's National Cooperative Growth Study (NCGS), which enrolled more than 40,000 GH recipients during 20 years of follow-up, showed that the leukemia risk was comparable to that of the general population when subjects with known risk factors for leukemia were excluded19). Another study from the NCGS reported 3 cases of de novo leukemia compared with the 5.6 cases expected in a theoretical, age-matched general population20).
2. Solid tumors
There has been concern that elevated endogenous levels of GH and IGF-I might be associated with an increased risk of certain solid tumors. Swerdlow et al.21) studied cancer incidence and mortality in 1,848 patients in the UK who were treated with human pituitary GH during childhood and early adulthood between 1959 and 1985. The incidence and mortality of colorectal cancer and the mortality of Hodgkin's disease initially increased after patients with an otherwise high risk of cancer were excluded. Since then, however, the validity of the results have been debated, because the current GH therapy regimen differs from what was used at the time8). All patients received standard GH doses given 2 or 3 times a week, and serum IGF-I levels were not monitored.
Tyden et al.22) reported 2 cases of de novo development of cancer in living kidney transplant recipients, although de novo development of cancer in renal transplants might be rare. According to the analysis from the Kabi International Growth Study (KIGS) and NCGS databases, however, only 2 cases of renal cell carcinoma in those who did not have renal disease were found among the 43,000 patients in the NCGS registry and 42,000 in the KIGS registry23). The number of malignancies seemed disproportionately high for the relatively small number of children who had chronic renal failure and who were receiving GH treatment8,23).
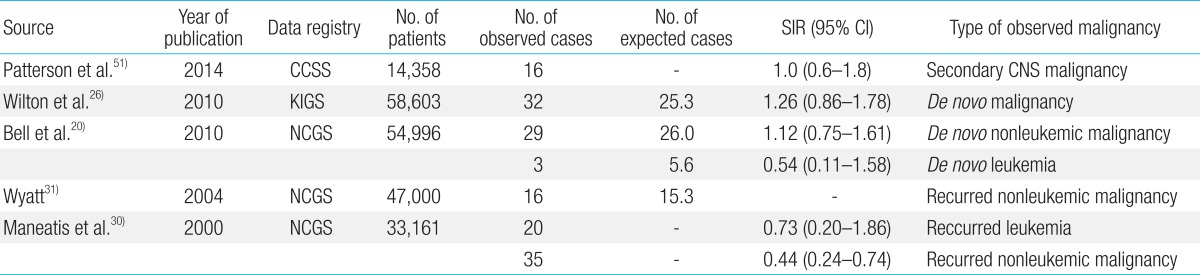
Tuffli et al.24) reported that the number of extracranial, nonleukemic neoplasms from 12,209 individuals who were treated with the recombinant GH was no greater than expected cases. Although 10 new cases of malignancies were noted, it was not a greater value than predicted, indicating that GH was not implicated in the occurrence of solid tumors. Another report from NCGS showed no evidence of an increase in the incidence of de novo intracranial tumors in children treated with GH25). Bell et al.20) reported, from a recent analysis of the NCGS registry, that de novo intracranial and extracranial malignancies were not significantly increased in patients without risk factors (29 confirmed vs. 26 expected). One of the most recent reports from the KIGS compared the incidence of cancer in the cohort to that in the general population by using the standardized incidence ratio (SIR)26). A total of 32 new malignant neoplasms were reported in 58,603 patients, versus the 25.3 expected (SIR, 1.26; 95% confidence interval [CI], 0.86-1.78). Again, patients treated with GH showed no statistically significant difference in cancer incidence compared with the expected number of cases in this study.
GH treatment and recurrence of malignancy
1. Leukemia
Survivors of childhood leukemia are at risk of developing complications, including growth failure, which may require GH treatment8). Patients with GH deficiency are particularly common because total body irradiation increases risk of growth failure, although GH deficiency can also result from solely chemotherapeutic regimens27). There has been concern that children treated with GH after therapy against leukemia may be at a higher risk of leukemia recurrence.
Leung et al.28) studied 47 patients who had received GH replacement therapy among 910 patients treated for acute lymphoblastic leukemia and examined recurrence rates at 7 years and 11 years after continuous hematologic remission. There was no statistical evidence that GH therapy was associated with leukemia relapse or development of a second malignancy.
The NCGS has monitored the safety of recombinant human GH since 1985. There have been 3 new cases of leukemia in children without known risk factors for developing leukemia and 5 cases in children with known risk factors; however, there was no evidence of an increased recurrence of leukemia29). Follow-up reports showed no evidence of increased incidence of leukemia among patients without previous risk factors30). Another investigator analyzed 47,000 patients representing 165,000 patient-years from the NCGS and found reassuring evidence that leukemia (de novo or relapse), extracranial nonleukemic neoplasms, and central nervous system (CNS) tumor recurrence were not associated with GH therapy31).
Sklar et al.32) investigated 122 acute leukemia survivors from among 13,539 subjects enrolled in the Childhood Cancer Survivor Study (CCSS), a cohort of 5-year survivors of childhood cancer. They found that the relative risk of recurrence was not increased compared to 4,545 children not treated with GH.
2. Brain tumors
GH deficiency is a common disease in patients with hypothalamic-pituitary tumors. The deficiency is caused either by the mass itself or by surgical or irradiation therapy to hypothalamopituitary axis9). Deficiency can also develop in those who have tumors far from the hypothalamopituitary axis, because cerebrospinal irradiation is often needed to treat these tumors33).
In 1985, Arslanian et al.34) reported the outcome of GH therapy in 34 children with brain tumors who developed hypopituitarism. Of the 34 patients with brain tumors and hypopituitarism, 24 received GH therapy. Eight of the 24 (33%) experienced tumor recurrence, compared with 3 of the 10 (30%) who did not receive GH. Clayton et al.35) reported similar results, namely that the late relapse rate of medulloblastoma and glioma was not altered by GH therapy, and the therapy might not increase the relapse rate of brain tumors.
Medulloblastoma is one of the most malignant childhood brain tumors. Survivors of medulloblastoma are, however, increasing as progress has been made in the treatment of the tumor36). From a retrospective analysis of 34 children treated with GH for medulloblastoma over 3 years, Chae et al.37) reported no cases of recurrence. Another retrospective review included 545 children with medulloblastoma, of whom 170 patients were treated with GH38). This review found that while GH treatment was underutilized in survivors of medulloblastoma, it was not associated with disease relapse.
Craniopharyngioma is a relatively common brain tumor derived from pituitary gland embryonic tissue in children39). Due to the morbidities associated with damage to the pituitary and hypothalamus from surgical removal and irradiation against craniopharyngioma, GH treatment is commonly needed after therapy to treat this tumor40). The recurrence rate of craniopharyngioma was 0.045/treatment year in 488 patients who were enrolled in the KIGS from 1988 to 1996, and the investigators concluded that GH treatment might be safe and effective in children with craniopharyngioma41). In the NCGS report, children receiving GH after treatment for craniopharyngioma had a recurrence rate of 6.4%, considerably lower than the estimates of 20%-25% in another report25,42).
Swerdlow et al.43) investigated 180 children with brain tumors treated with GH, and 891 children not treated with GH during 1965-1996. The relative risk of first recurrence in GH-treated patients decreased after adjusting for potentially confounding prognostic variables (0.6; 95% CI, 0.4-0.9). The relative risk of mortality also decreased (0.5; 95% CI, 0.3-0.8). There was no significant trend in relative risk of recurrence with cumulative time for which GH treatment had been given or with time elapsed since GH treatment started. Another study showed, using surveillance imaging, that hypothalamopituitary tumor recurrence in GH-treated patients was as low as in non-GH-treated patients44). Only 1 patient among 100 consecutive patients showed evidence of slight intrasellar tissue enlargement using pituitary imaging at 6 months. GH replacement was continued, and there was no further change between 6 and 12 months, though the follow-up duration was short and may not be conclusive evidence against recurrence.
GH treatment and risk of secondary malignancy
Childhood cancer survivors might be at increased risk for secondary malignancies compared with the general population45). Assessing their risk of second and subsequent malignancies during long term follow-up is thus very important.
Meadows et al.46) investigated 14,358 cohort members in the CCSS and analyzed SIRs for occurrences of a second malignant neoplasm. The 30-year cumulative incidence of second malignant neoplasm was 9.3%, and the risk of subsequent neoplasms remained elevated for more than 20 years of follow-up for all diagnoses of primary childhood cancer. Another report from the CCSS showed that the risk of sarcoma was more than 9 folds greater among childhood cancer survivors than among the general population47). Ergun-Longmire et al.48) analyzed the cohort and reported that the rate of GH-treated survivors developing secondary neoplasms was 2.1 folds greater compared with non-GH-treated survivors; however, the risk appeared to decrease as follow-up time extended. Using large cohort follow-up studies, Carel and Butler49) suggested that children treated with GH following childhood cancer treatment might not have a greater number of relapses, but that there might be a higher incidence of secondary tumors in the early years of GH therapy.
There are, however, some more reassuring data. Neglia et al.50) analyzed 13,581 children from a retrospective cohort drawn from US and Canadian institutions who were diagnosed with common cancers before the age of 21 and survived at least 5 years. Twenty years after the childhood cancer diagnosis, the estimated cumulative incidence of second malignancy was 3.2%, and only 1.88 excess malignancies occurred during follow-up. Sklar et al.32) studied 172 brain tumor survivors from among 13,539 survivors enrolled in the CCSS. The relative risk of disease recurrence was 0.83 (95% CI, 0.37-1.86) for survivors who had been treated with GH, and risk was not increased for any of the major cancer diagnoses.
Most recently, Patterson et al.51) analyzed 12,098 pediatric cancer survivors from the CCSS and reported the incidence of meningioma, glioma, and other solid CNS tumors. The adjusted rate of CNS tumor development in patients treated with GH compared with untreated survivors was 1.0 (95% CI, 0.6-1.8). Overall, there was no statistically significant increase in risk of the occurrence of solid CNS tumors associated with GH exposure. Major recent studies of growth hormone treatment and malignancy risk from large data registries were summarized in Table 1.
Levels of circulating IGF-I might be associated with an increased risk of common cancers, although the association remains unclear52), as there have been few large cohort studies studying the relationship between GH dosage, IGF-I levels, and risk of tumor recurrence. A meta-analysis and systematic review showed that high concentrations of IGF-I were associated with an increased risk of prostate cancer and premenopausal breast cancer, but the associations were modest52). Nonetheless, monitoring and maintaining IGF-I levels within a normal range might be important, and titrating GH doses to target IGF-I levels (IGF-I based dosing) could be a reasonable approach in this high risk GH-treated group49).
Conclusions
The benefits of GH treatment for growth and metabolism in children with GH deficiency are well defined, and replacement therapy with GH is recommended53). Despite theoretical concerns about the effect of GH on tumor development, this review of various clinical and epidemiological studies demonstrated that there is no clear evidence of a causal relationship between GH treatment in patients with GH deficiency and tumor development. Nonetheless, a small number of studies have reported that childhood cancer survivors who have received GH treatment have a small increased risk of de novo cancer and second malignant neoplasm. Therefore, regular follow-ups and careful examination for the development of cancer should be required in those who receive GH treatment. Continued surveillance for an extended period is essential to monitor further potential changes.
Notes
No potential conflict of interest relevant to this article was reported.