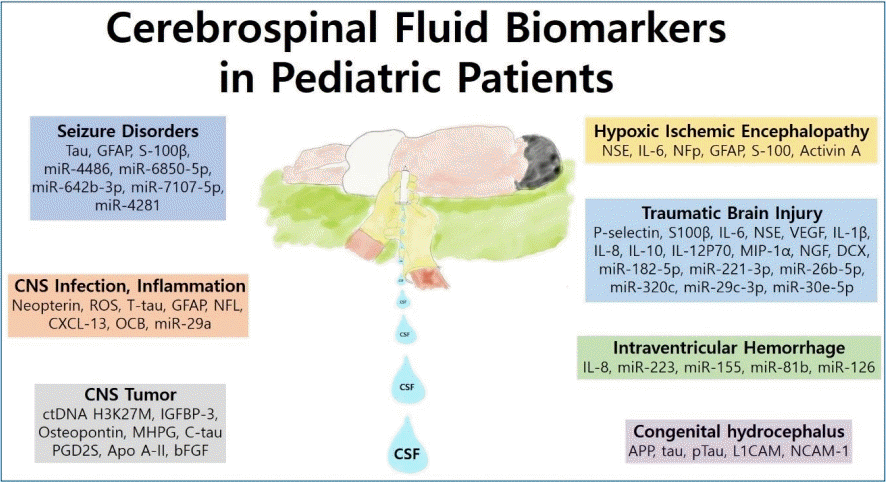
Promising candidate cerebrospinal fluid biomarkers of seizure disorder, infection, inflammation, tumor, and traumatic brain injury in pediatric patients
Article information
Abstract
Cerebrospinal fluid (CSF) is a dynamic metabolically active body fluid that has many important roles and is commonly analyzed in pediatric patients, mainly to diagnose central nervous system infection and inflammation disorders. CSF components have been extensively evaluated as biomarkers of neurological disorders in adult patients. Circulating microRNAs in CSF are a promising class of biomarkers for various neurological diseases. Due to the complexity of pediatric neurological disorders and difficulty in acquiring CSF samples from pediatric patients, there are challenges in developing CSF biomarkers of pediatric neurological disorders. This review aimed to provide an overview of novel CSF biomarkers of seizure disorders, infection, inflammation, tumor, traumatic brain injuries, intraventricular hemorrhage, and congenital hydrocephalus exclusively observed in pediatric patients.
Key message
· Pediatric cerebrospinal fluid (CSF) components have been extensively evaluated as biomarkers of various neurologic diseases.
· Several promising candidate CSF biomarkers, including Tau, glial fibrillary acidic protein, neuron-specific enolase, S100β, and interleukins, have been studied in pediatric patients with seizure disorders, central nervous system infections, inflammation, tumors, hypoxic-ischemic encephalopathy, traumatic brain injuries, intraventricular hemorrhage, and congenital hydrocephalus.
· Circulating microRNAs in the CSF are a promising class of biomarkers for various neurological diseases.
Graphical abstract. Summary of cerebrospinal fluid (CNS) biomarkers of neurologic disorders in pediatric patients.
Introduction
Cerebrospinal fluid (CSF) analysis has been widely used to diagnose or exclude central nervous system (CNS) infection disorders. These CSF examinations include CSF/serum albumin ratio, cell count, immunoglobulin (Ig) G (IgG), IgM, lactate, and culture [1]. In addition, because disorders of intracranial pressure can result in sudden or progressive headache, CSF pressure has been used to evaluate headache [2]. Intracranial hypertension and hypotension syndromes can cause headaches [2]. The intraventricular catheter method is the gold standard measurement for determining intracranial pressure. However, lumbar puncture (LP) is less invasive [3]. Although rare in children, headache can occur as a complication of LP [4]. The correct analysis of CSF is crucial in diagnosing infectious and inflammatory conditions including the brain, spinal cord, and meninges. Because of the close proximity between the CSF and the brain, CSF is a good source for biomarkers that reflect pathological processes within the CNS [1].
Since CSF is more easily acquired in adult patients than pediatric patients by LP, CSF components have been extensively evaluated as biomarkers of neurologic disorder including Alzheimer disease, amyotrophic lateral sclerosis, traumatic brain injury (TBI), and adult-onset normal-pressure hydrocephalus [5-9]. However, because of the invasiveness of LP, it is difficult to acquire CSF from pediatric patients and almost impossible to acquire CSF from a healthy child. This makes it difficult to identify CSF biomarkers for pediatric neurological disorders. MicroRNAs (miRNAs) in the CSF were recently shown to be a promising class of biomarkers for various neurological diseases, including CNS tumors, Alzheimer disease, multiple sclerosis (MS), Parkinson disease, and status epilepticus [10-14]. These miRNAs and short interfering RNAs are well-known small RNAs that play a major role in RNA interference [15]. In particular, miRNAs are endogenous single-stranded noncoding RNAs with 18–23 nucleotides capable of playing important roles in targeting mRNAs for cleavage or translational repression [16,17]. Altered levels of circulating miRNAs have been seen in many diseases [18,19]. Also, miRNAs mostly function in the intracellular compartment, although cell-free circulating miRNAs have been detected in most extracellular fluids, including the CSF [20]. Although only a few CSF studies in pediatric patients to date have aimed to identify biomarkers for diagnosing and managing neurological disorders, various other studies are beginning to emerge. Only a few comprehensive review articles to date have reported on CSF analyses of pediatric neurological disorders. This review will focus on promising candidate CSF biomarkers of neurological disorders exclusively observed in pediatric patients.
CSF sampling, preparation, storage, and interpretation
CSF is a dynamic metabolically active body fluid that has many important roles [21]. The CSF is an ultrafiltrate of plasma that is normally composed of 99% water [22]. It also contains electrolytes, glucose, enzymes, proteins, and a few white blood cells [22]. CSF fills the subarachnoid space; in children aged 4–13 years, the static CSF volume is 65–150 mL [21]. The neonate choroid plexus produces approximately 25 mL/day of CSF, while the circulating volume is approximately 50 mL [23]. The CSF production rate reaches 14–36 mL/hr, and the total volume increases to approximately 150 mL in adults [21]. It is crucial to precisely analyze CSF samples to acquire valid information [1]. Using low-absorbing polypropylene tubes is recommended and because there is a craniocaudal gradient for some CSF biomarkers, and it is necessary to collect a standardized volume of CSF [1]. Changes in the proteome have been observed in uncentrifuged CSF samples left at room temperature for as few as 30 minutes [24]. The recommended ideal spinning centrifugation condition is spinning at 2,000 × g for 10 minutes, preferably in a cooled condition, or 400–450 × g when the cells are to be preserved for analysis [25]. Time must be minimized for transport to the laboratory and the samples must be cooled on ice during transport [25]. Blood contamination can disturb the interpretation of samples; thus, traumatic LP should be avoided by not overshooting the needle insertion, as there is an extensive venous plexus lining the ventral wall of the subarachnoid space [1]. If trauma occurs during the LP, the CSF sample must be centrifuged immediately and the clear CSF transferred to a new tube [1]. Interpreting CSF biomarkers is complicated in pediatric patients, especially those in the first year of life, because age reference values are different. CSF levels of tau, glial fibrillary acidic protein (GFAP), and the CSF/albumin ratio is known to differ among ages due to normal development of the CNS [26]. Protein concentrations of CSF are higher in newborns and older adults, lowest in children, and increase again in adolescence and adulthood [27].
CSF biomarkers of pediatric neurological disorders
1. Seizure disorders
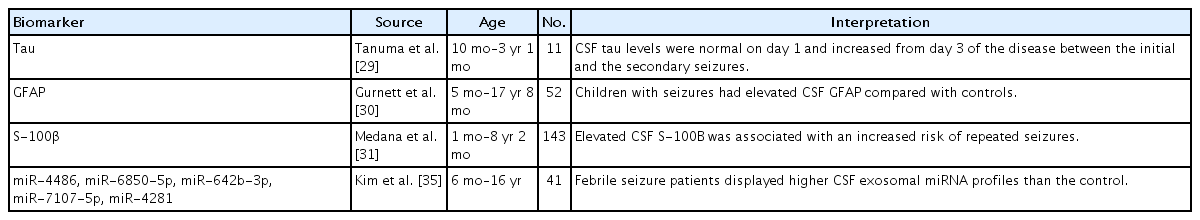
Tau is a marker associated with axonal degeneration and widely used in dementia investigations [1]. Tau protein is typically found in cortical axons and stabilizes microtubules [28]. Elevated total tau level in the CSF was observed in pediatric patients with acute encephalopathy with biphasic seizures but not in those without encephalopathy and only febrile seizures [29]. Tau levels are high in newborn infants, possibly due to neuroaxonal plasticity, and decrease during the first years of life [26]. Elevated CSF levels of GFAP and S-100 calcium-binding protein beta (S-100β) were found in children with seizures (Table 1) [29-31]. GFAP is an intermediate filament protein that is produced in astrocytes and ependymal cells of the CNS [32]. GFAP in the CSF is increased in children with white-matter disorders and can be measured to track gliosis [33]. S-100β is also a glial marker that is expressed by astrocytes and promotes astrocyte proliferation, neuritis growth, and neuronal development [1,34].
Few studies have shown a relationship between seizures and CSF miRNA. Furthermore, febrile seizure patients displayed higher CSF exosomal miRNA profiles than controls, and these altered miRNA profiles appeared related to complex febrile seizures [35]. These highly expressed miRNAs included miR-4486, miR-6850-5p, miR-642b-3p, miR-7107-5p, and miR-4281 (Table 1) [35].
2. CNS infection and inflammation
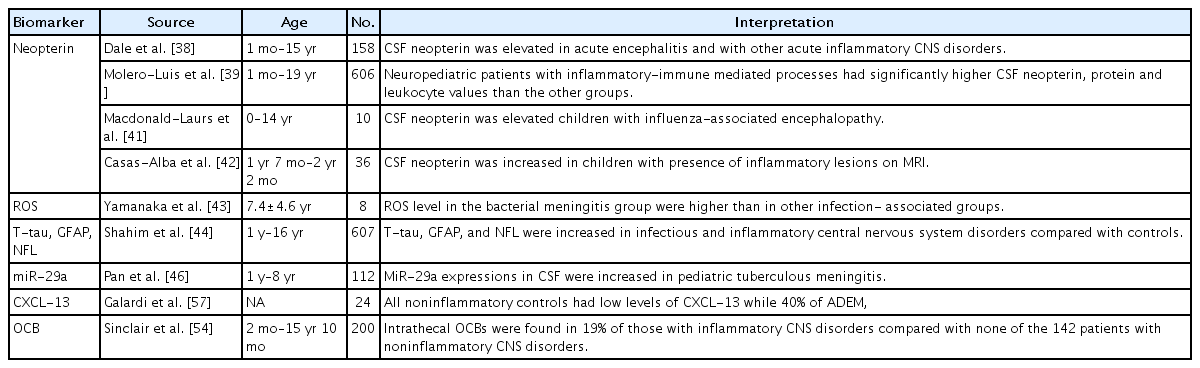
CSF/serum albumin ratio and CSF cell counts, culture, lactate, and IgG or IgM measurements are basic CSF tests that have been used to diagnose infectious and inflammatory CNS disorders [1]. To define the etiology of CNS infections, next-generation sequencing is gradually being tested in CSF samples of pediatric patients with encephalitis, meningoencephalitis, and meningitis [36]. However, the diagnostic value of next-generation sequencing of the CSF of pediatric patients is difficult to quantify [36]. One observational retrospective study analyzed 3 CSF biomarkers including neopterin, total proteins, and leukocytes in pediatric patients with neuroinflammatory disorders including viral meningoencephalitis, bacterial meningitis, and acquired-immune mediated disorders (Table 2) [37]. In the canonical discriminant analysis of these 3 biomarkers, the value of CSF neopterin was the highest in the viral and bacterial infection groups [37]. Neopterin is known to be elevated under inflammatory conditions and independently produced in the brain [38-40]. It has been suggested that microglia and astrocytes produce neopterin since these cells respond to interferon-gamma [40]. Peripheral neurons including dorsal root ganglia neurons also produce neopterin under inflammatory conditions [40]. An increased level of neopterin in the CSF has been reported in several diseases including acute bacterial and viral infections and chronic neuroinflammatory diseases [38,39,41,42].
One study assessed the validity of CSF oxidative status in pediatric patients with CNS diseases [43]. A total of 87 pediatric patients were enrolled in the study, and the mean level of reactive oxygen species in the bacterial meningitis group was significantly higher than that of the other infection-associated group [43]. Another study analyzed total tau, GFAP, and neurofilament light (NFL) levels in CSF of pediatric patients [44]. This study showed that total tau, GFAP, and NFL levels were increased in patients with infectious and inflammatory CNS disorders compared with controls [45]. NFL is a structural protein that is rich in myelinated axons and elevation of NFL is a indicator of white matter axonal injury [33].
A study of 122 children with tuberculous meningitis showed that miR-29a expression in the peripheral blood and CSF was increased compared with the control group [46]. The authors suggested that the miR-29a level of the peripheral blood and CSF could be a biomarker for diagnosing tuberculous meningitis [46].
CSF studies in acute disseminated encephalomyelitis (ADEM) are notable for their lack of confirmatory features [47]. CSF leukocyte counts have are reportedly normal in 42%–72% of pediatric patients with ADEM [47]. Pleocytosis is typically mild, with a high percentage of lymphocytes and monocytes [48,49]. An elevated CSF IgG level has been reported in several cohorts, and oligoclonal bands (OCBs) are rarely detected in the CSF of ADEM patients [50-53]. Sinclair et al. [54] analyzed OCBs in CSF from 200 children who underwent CSF investigations of their neurological condition and found intrathecal OCBs (those restricted to the CSF) in 11 of 58 (19%) of those with inflammatory CNS disorders versus none of the 142 patients with noninflammatory CNS disorders. The authors concluded that OCBs are a useful but nonspecific biomarker of CNS inflammation of multiple causes [54].
In MS, LP is routinely performed to obtain supportive evidence of CNS inflammation [55]. In about 60% of pediatric patients with MS, routine analysis of CSF reveals normal findings [56]. The remainder of the patients had pleocytosis, elevated protein levels, increased IgG levels, and OCBs [45]. One study analyzed chemokine (C-X-C motif) ligand 13 (CXCL-13) levels in children with demyelinating disease and showed that 40% of ADEM, 22.2% of clinically isolated syndrome, and 62.5% of MS children had high CXCL-13 levels versus noninflammatory controls [57].
3. CNS tumors
Pediatric CNS tumors are the leading cause of cancer-related mortality in childhood [58]. The diagnosis of CNS tumors relies on biopsy of the tumor tissue and surgical intervention [59]. There is a need for less invasive methods to diagnose and characterize CNS tumors. Because of the blood-brain barrier, blood is not ideal for evaluating CNS tumors [60]. Moreover, blood is not a suitable for evaluating metastatic CNS tumors if the patients have coexisting systemic tumors [61]. In contrast to the blood, the CSF is in direct contact with the CNS and a suitable source of biomarkers from CNS tumors [60].
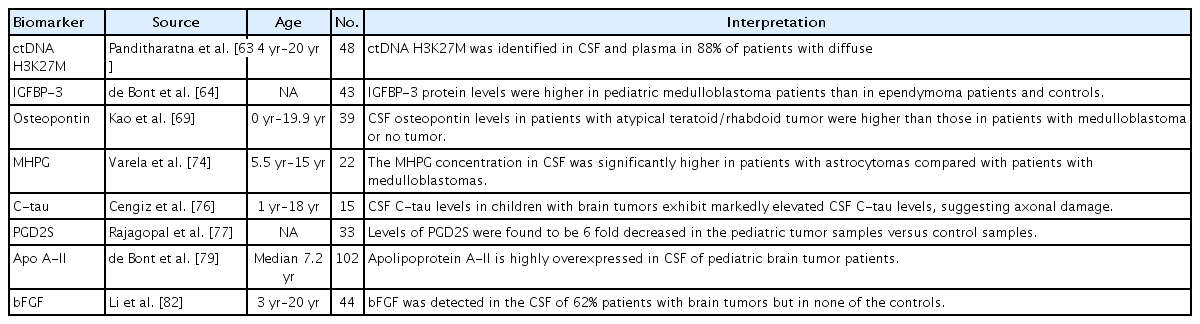
A number of tumor-derived biomarkers have been demonstrated in the CSF of adult patients with CNS tumors, including circulating tumor DNA (ctDNA), miRNA, and metabolites (Table 3) [60,62]. However, only a few CSF biomarker studies have examined pediatric patients. Panditharatna et al. [63] identified ctDNA H3K27M in the CSF and plasma of 88% of pediatric patients with diffuse midline glioma. The authors concluded that the CSF and plasma were appropriate for the detection of ctDNA and provided a molecularly based tool for tumor characterization [63]. One study analyzed protein expression levels of insulin-like growth factor (IGF) and IGF binding protein (IGFBP) in the CSF of pediatric medulloblastoma and ependymoma patients [64]. The IGF system plays a major role in regulating CNS development and is involved in the pathogenesis of brain tumors [65-68]. The IGFBP-3 levels were higher in pediatric medulloblastoma patients than in ependymoma patients and controls [64]. The authors suggested that the CSF IGFBP-3 level of medulloblastomas was a potential marker of residual disease [64].
A study of a cohort of 39 patients showed that the mean plasma and CSF osteopontin levels in atypical teratoid and rhabdoid tumors were higher than those in hydrocephalus, medulloblastoma, and epilepsy patients [69]. A primary CNS atypical teratoid rhabdoid tumor is an extremely malignant neoplasm in pediatric patients [70]. Osteopontin is a bone matrix glycoprotein that has a role in mineralization and bone resorption [71]. It also contributes to angiogenesis or neovascularization via the expression of vascular endothelial growth factor [71-73]. Varela et al. showed an association between monogenic amines in the ventricular CSF of pediatric patients with posterior fossa tumors [74].
Biogenic amines including dopamine, epinephrine, norepinephrine, and serotonin are involved in the regulation of various neuronal functions, while changes in the monoamine levels of the CSF have been detected in multiple disorders [74,75]. They reported that the 3-methoxy-4-hydroxyphenylglycol level of the CSF was significantly higher in pediatric patients with astrocytoma than in those with medulloblastoma [74]. A prospective clinical observational study demonstrated that children with newly diagnosed brain tumors exhibit markedly elevated CSF cleaved tau levels, which were suggestive of axonal damage [76].
Prostaglandin D2 synthase (PGD2S) levels were six-fold lower in the pediatric tumor samples versus control samples in study by Rajagopal et al. [77]. The authors speculated that the reduction is a host response to the presence of the tumor [77]. PGD2S is a glycoprotein that is abundant in the CSF and is synthesized and secreted by both glial cells and the choroid plexus [78]. De Bont et al. [79] showed that apolipoprotein A-II (APO A-II) was highly overexpressed in the CSF of pediatric brain tumor patients, which is most likely is associated with a disrupted blood-brain barrier. APO A-II is the second most abundant human high-density lipoprotein apolipoprotein and synthesized predominantly in the liver [80]. APO A-II is known to influence the metabolism of high-density lipoprotein and glucose and was recently linked to malignancies [81]. The CSF level of basic fibroblast growth factor (bFGF) was also increased in pediatric patients with brain tumors. 82) Moreover, bFGF is a widely distributed angiogenic molecule, and most cells produce it or have receptors for it [83].
4. Hypoxic-ischemic encephalopathy
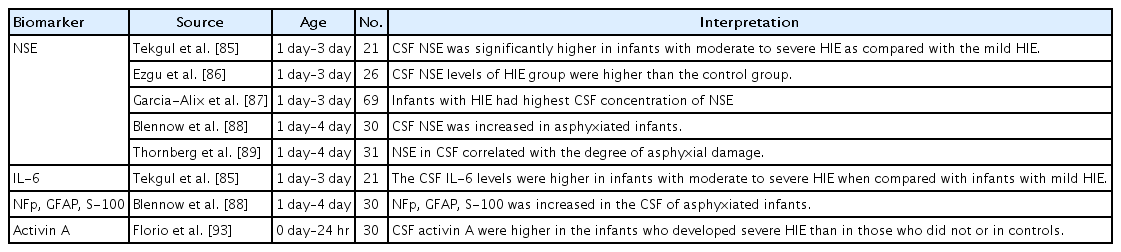
Hypoxic-ischemic encephalopathy (HIE) contributes to worldwide major perinatal mortality and long-term disability in neonates [84]. In neonates with HIE, CSF levels of neuron-specific enolase (NSE) have been shown to play an important role as a marker of brain damage extent and enabled the prediction of the outcomes of infants with HIE in the prehypothermia era (Table 4) [85-89]. NSE is a glycolytic enzyme, and structural damage to neuronal cells causes leakage of NSE into the extracellular compartment [90]. Interlukin-6, neurofilament protein, GFAP, and protein S-100 are known to be associated with neonatal HIE [85,88]. Therapeutic hypothermia appears to lower CSF NSE levels in infants with HIE; however, the predictive value of CSF NSE for neurodevelopmental impairment at 12 months of age is not affected by cooling [91]. One study showed that CSF levels of NSE predicted brain damage severity in newborns with hypothermiatreated HIE [92]. Increased CSF levels of activin A were detected in term neonates with perinatal asphyxia, and the highest concentrations were found among neonates with the most severe HIE [93]. Activin A is a member of the transforming growth factor-beta family, and it enhanced expression is known to represent a common response to acute neuronal damage of various origins [94].
5. Traumatic brain injury
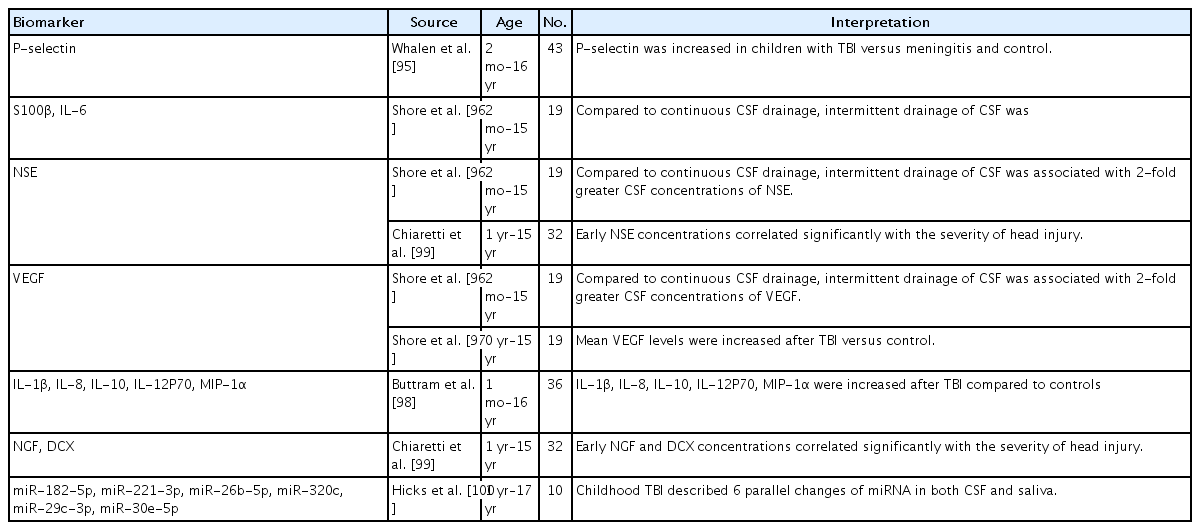
In one study, the levels of platelet selectin (P-selectin), an adhesion molecule associated with ischemia and reperfusion, were elevated in pediatric patients’ CSF with TBI versus those with meningitis or controls (Table 5) [95]. Increased blood-brain barrier permeability may contribute to the increased CSF P-selectin levels of pediatric patients with TBI [95]. Platelets are a major source of P-selectin; therefore, hemorrhage might cause the elevated P-selectin level in the CSF of TBI patients [95]. Shore et al. [96] reported that, compared to continuous CSF drainage, intermittent drainage of the CSF was associated with 2-fold greater CSF concentrations of NSE, s100B, interleukin (IL)-6, and vascular endothelial growth factor (VEGF) [96]. CSF VEGF levels were increased after TBI versus control in another study [97]. VEGF is known to be neuroprotective in several experimental brain injury models, and its levels are increased in the brain after TBI in humans and experimental animals [97]. CSF levels of cytokines such as IL-1β, IL-8, IL-10, IL-12P70, and macrophage inflammatory protein-1 alpha were also increased after TBI compared to controls in another study [98]. Early nerve growth factor and doublecortin concentrations also seemed to be correlated with TBI severity [99].
An miRNA study of the CSF and saliva after childhood TBI described 6 parallel changes in miRNA (miR-182-5p, miR-221-3p, miR-26b-5p, miR-320c, miR-29c-3p, and miR-30e-5p) in both [100].
6. Intraventricular hemorrhage
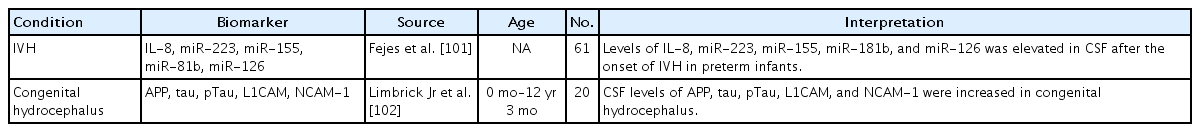
Intraventricular hemorrhage (IVH) has a high risk of neonatal mortality and later neurodevelopmental impairment in preterm infants [101]. One study analyzed proinflammatory cell-free miRNA levels in the CSF of 47 preterm infants with grade III or IV IVH versus controls and reported that the levels of miR-223, miR-155, miR-181b, miR-126, and IL-8 were elevated in the CSF after the onset of IVH versus controls (Table 6) [101]. The authors claimed that hemorrhage-induced CSF miRNA levels reflected inflammatory conditions as potential biomarkers in cases of preterm IVH [101].
7. Congenital hydrocephalus
The variety of etiologies of pediatric hydrocephalus creates additional complexities in biomarker analyses [9]. A recent study of the CSF of infants with untreated congenital hydrocephalus showed a significant increase in CSF amyloid precursor protein (APP) (Table 6) [102]. In this study, CSF levels of APP and its derivative isoforms sAPPα, sAPPβ, Aβ42, tau, phosphorylated tau, L1 neural cell adhesion molecule, and neural cell adhesion molecule but not aquaporin 4 or total protein were increased in untreated congenital hydrocephalus, particularly in children ≤1 year of age, and CSF sAPPα levels showed the strongest relationship with congenital hydrocephalus [102]. AAP is released in the setting of axonal injury, and hydrocephalus-related ventriculomegaly causes axonal pathology [103]. In addition, APP and its derivative isoforms are trophic factors with important roles in synaptogenesis and other aspects of neurodevelopment [104].
Challenges of identifying CSF biomarkers in pediatric neurologic disorders
Due to the difficulty acquiring a healthy child’s CSF, only CSF from different conditions could be controls in CSF biomarker studies in pediatric disorders. Heterogeneity of pediatric neurological disorders is also a challenge for developing a CSF biomarker, and the fact that there are no reference values for many biomarkers in different age ranges, it is difficult to identify CSF biomarkers in children. Large-scale multi-institutional CSF studies, including network repositories, are needed to address these challenges. Advances in laboratory techniques that minimize the amount of CSF needed to analyze biomarkers should help in the development of CSF biomarkers for pediatric neurological disorders.
Conclusion
In pediatric neurological disorders, several promising CSF biomarkers that may be used to diagnose and monitor the effects of therapy. Although significant progress has been made in identifying CSF biomarkers of pediatric neurologic disorders, no single pathognomonic novel biomarkers are available for any pediatric neurologic disorders. Due to the complexity of pediatric neurological disorders and difficulty acquiring CSF from pediatric patients, identifying CSF biomarkers is challenging. Proper CSF laboratory standardization should be performed, and large-scale investigations should be performed to overcome these challenges.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.