Ambient air pollution and allergic diseases in children
Article information
Abstract
The prevalence of allergic diseases has increased worldwide, a phenomenon that can be largely attributed to environmental effects. Among environmental factors, air pollution due to traffic is thought to be a major threat to childhood health. Residing near busy roadways is associated with increased asthma hospitalization, decreased lung function, and increased prevalence and severity of wheezing and allergic rhinitis. Recently, prospective cohort studies using more accurate measurements of individual exposure to air pollution have been conducted and have provided definitive evidence of the impact of air pollution on allergic diseases. Particulate matter and ground-level ozone are the most frequent air pollutants that cause harmful effects, and the mechanisms underlying these effects may be related to oxidative stress. The reactive oxidative species produced in response to air pollutants can overwhelm the redox system and damage the cell wall, lipids, proteins, and DNA, leading to airway inflammation and hyper-reactivity. Pollutants may also cause harmful effects via epigenetic mechanisms, which control the expression of genes without changing the DNA sequence itself. These mechanisms are likely to be a target for the prevention of allergies. Further studies are necessary to identify children at risk and understand how these mechanisms regulate gene-environment interactions. This review provides an update of the current understanding on the impact of air pollution on allergic diseases in children and facilitates the integration of issues regarding air pollution and allergies into pediatric practices, with the goal of improving pediatric health.
Introduction
Allergic disorders, particularly asthma, have emerged as a major worldwide public health issue due to their increasing prevalence and the associated growth in health care costs1). The prevalence of allergic disorders has been increasing over the past 30 to 40 years2). The International Study of Asthma and Allergies in Childhood (ISAAC) reported rising trends in the prevalence of allergic disorders worldwide, particularly in highly developed countries3), and the Korean ISAAC study revealed a similar rising trend in the prevalence of atopic disorders, which suggests that the situation in Korea is similar to that of other countries4-6). In Korea, asthma-related costs ranked number one among childhood diseases, and the socio-economic burden of the disorder is increasing in all age brackets7).
This increasing trend suggests that both environmental and genetic factors may play an important role in allergies. Improved hygiene, dietary habits, and increased air pollution are believed to have caused this trend8-10). Among environmental factors, air pollution is considered an important factor because people have begun to live in more highly populated and urbanized areas, where traffic largely contributes to air pollution. The World Health Organization (WHO) also found that the air quality in large cities in many developing countries is remarkably poor and that very large numbers of people in those countries are exposed to ambient concentrations of air pollutants well above the WHO guidelines for air quality11). Special concern should be given to children because they may be particularly vulnerable to air pollution due to their underdeveloped respiratory and immune systems12) and the fact that they inhale more air per square meter of body surface than adults, which would increase their exposure to air pollutants13).
Many studies show a potential association between exposure to traffic exhaust fumes and allergic diseases in children14-17). For example, exposure to a high level of air pollutants from traffic increases the prevalence of asthma symptoms and physician-diagnosed asthma14).
Health professionals need to know about environmental factors affecting health and have to inform individuals at high risk of such health effects in order to minimize short-and long-term health consequences. Therefore, the aim of this review is to provide an update of the current understanding on the association of air pollution with childhood allergic disease and the possible mechanisms involved. This review will help pediatricians to integrate issues regarding air pollution and allergy into their practices to improve pediatric health.
Association of air pollution and childhood allergic disease: epidemiologic studies
Air pollutants have various adverse effects on early life, and some of the most important harmful effects of these pollutants are perinatal disorders, infant mortality, respiratory disorders, allergy, increases in oxidative stress, and endothelial dysfunction. The late-onset effects of air pollution in early life may be related to many chronic diseases later in life18-20).
Recent epidemiologic, human, and animal model studies have demonstrated that diesel exhaust particulates (DEPs) from traffic, major source of air pollution, increase airway inflammation and can exacerbate and initiate asthma and allergy. Because of the complex nature of diesel exhaust composition, obtaining an accurate measure of exposure has been difficult. Therefore, most early studies have shown that residing near busy roadways is associated with increased asthma hospitalization, decreased lung function, and increased prevalence and severity of wheezing and allergic rhinitis21,22). Until recently, proximity to main roads has been used as a proxy for exposure to air pollution. For example, a recent study showed that the shorter the distance of the residence from the nearest main road during pregnancy was associated with an increased risk of doctor-diagnosed asthma and atopic dermatitis23). A preliminary study by our group also revealed that, when children lived nearer to traffic-congested roads, they experienced an increase in the development of wheezing and airway hyper-responsiveness24).
In epidemiological studies, how to assess exact exposure to air pollution has been an important issue. Assessment of an individual's exposure to air pollution is complicated by various factors such as climate, configuration of ground, and housing patterns, making estimations distorted from real values.
Recently, prospective studies using more accurate measurement of individual exposure to air pollution have been conducted and have provided definitive evidence of the impact of air pollution on allergic diseases. These studies used validated methods and land-use regression (LUR) models to estimate the annual average air pollution concentrations at residence locations with a prospective study design. An international collaborative study on the risks of development of childhood asthma and other allergic diseases ("risk assessment of exposure to traffic-related air pollution for the development of inhalant allergy, asthma and other chronic respiratory conditions in children" or Traffic-Related Air Pollution on Childhood Asthma [TRAPCA]) composed of 3 birth cohort studies from Germany, Holland, and Sweden, followed children until the age of 4 or 6 years and suggested a positive relationship between traffic-related pollution and physician-diagnosed asthma16,25,26). Recently, extended follow-up revealed a positive association between traffic-related pollution levels at the birth address and incidence of asthma during the first 8 years of life14).
A recent systemic review evaluated 13 papers based on data from 9 cohorts27). The outcomes varied, however, according to the age of the child. Nevertheless, the consistency in the results indicates that traffic exhaust contributes to the development of respiratory symptoms in healthy children. Regarding the development of allergic sensitization, the results were different according to the child's age and country. These results imply that the effects of air pollution may be different among individuals with specific genetic polymorphisms. Therefore, further studies need to consider gene and environmental (air pollution) interactions in order to identify children vulnerable to air pollution.
Methods for assessing an individual's exposure to air pollution
It is not possible to use individual measuring devices for every subject in epidemiological studies; therefore, exposure levels are estimated using the geometric information systems (GISs). The Kriging method28) is a weighted combination of monitor values and uses spatial autocorrelation among data to determine weights rather than assuming a function of inverse distances. Kriging predicts individual levels corresponding to residential locations after estimating the parameters of a model of the spatial surface of concentrations of air pollutants. This method was used to estimate individual exposure levels in the Children's Health and Environmental Research (CHEER) study, a nationwide prospective cohort study investigating environmental effects on children's health, and our group reported the results describing associations of ozone (O3) with asthma, allergic rhinitis, and allergic sensitization17).
Exposure at the home address has also been estimated using dispersion models29) or LUR models30). While atmospheric dispersion models require meteorological information and emission data (i.e., emission factors for vehicle fleets), the LUR models explain existing contrasts in monitored concentrations using, for example, road type and population density. Briefly, GIS data on traffic, roads, and population density in the vicinity of each monitoring location are collected, then regression models are developed to relate the annual average concentrations measured at the monitoring stations with the GIS variables. The LUR model was originally developed as part of the TRAPCA study and has been validated by these 3 cohort studies as previously described16,25,26).
Air pollution monitoring system in Korea
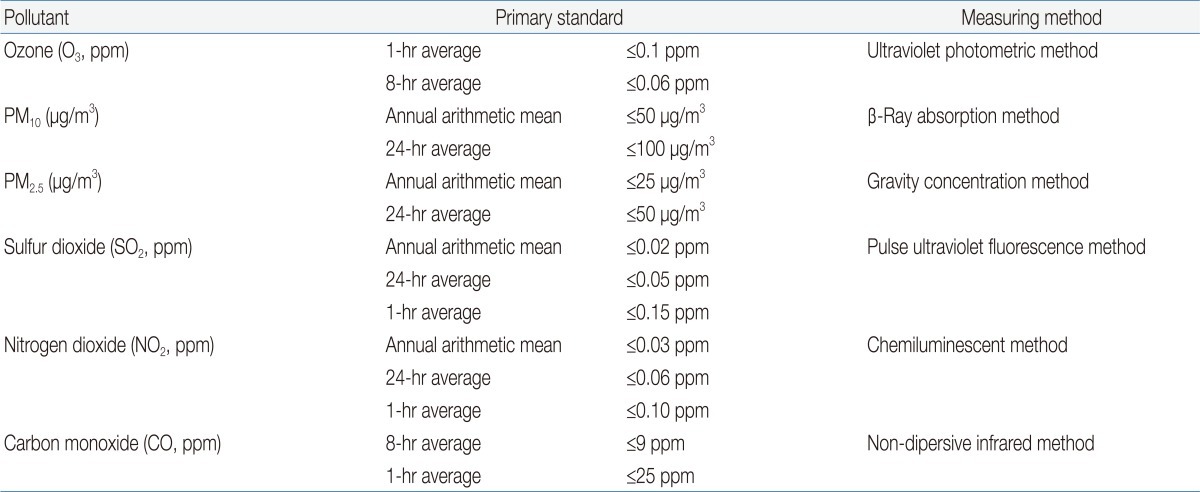
The department of environment in the republic of Korea operates an air pollution monitoring network, which is concerned with the installation and operation of air pollutant monitoring equipment to identify nationwide air pollution. Air pollution monitoring data are routinely collected at monitoring stations nationwide. The hourly data available for gaseous pollutants and particulate matter (PM) in monitoring stations are used to determine daily, monthly, and yearly averages. Data collected by the air pollution monitoring network are processed by the National Ambient Air Monitoring Information System (collaboratively operated by the Ministry of Environment and the Environment Management Corporation, Fig. 1) and are utilized as resources for the regulation of the atmosphere in order to assess air quality, analyze the effectiveness of environmental regulations, and protect the health of citizens by determining National Ambient Air Quality Standards for air pollutants (Table 1). Real-time air quality and periodic data can be found on at http://www.airkorea.or.kr.

National Ambient Air Monitoring Information System (NAMIS) in Korea. Program to gather, screen and statistically process data from nationalwide air pollution monitoring network through exclusive circuits.
Air pollution and its effects on childhood allergic diseases: sources and possible mechanisms
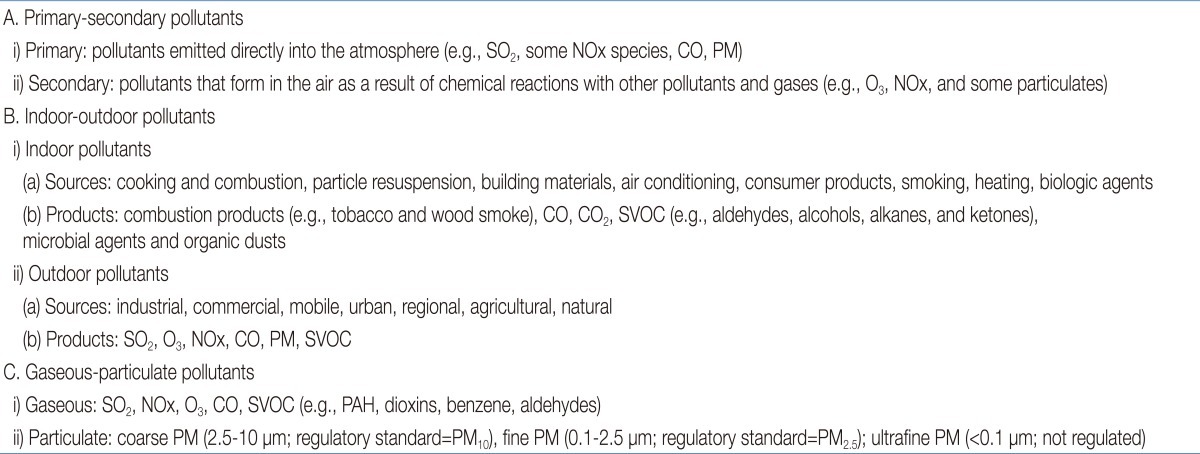
Air pollution arises from a variety of sources, of which the combustion of fossil-fuels, primarily from motor vehicles, in addition to power stations and factories, is the principal source. Air pollutants can be classified by their source, chemical composition, size, and mode of release into indoor or outdoor environments. The examples listed in Table 2 distinguish between primary and secondary, indoor and outdoor, and gaseous and particulate pollutants. Pollutants directly emitted into the atmosphere are known as primary pollutants, whereas pollutants that form as a result of chemical reactions with other pollutants or atmospheric gases are known as secondary pollutants.
Motor vehicles pollute the air through tailpipe exhaust emissions and fuel evaporation, and road traffic is the main contributor to air pollution. Traffic-related air pollutants consist of PM, ground-level O3, carbon monoxide, sulfur oxides, and nitrogen oxides; of which, particle pollution and ground-level O3 are the most widespread health threats. A brief summary of these common pollutants and their effects on childhood allergic disease is provided here.
1. PM
PM is a heterogeneous mixture of very small particles and liquid droplets suspended in air. Based on the size of the particles, PM pollution is classified into 3 categories. Coarse PM (aerodynamic diameter, 2.5 to 10 µm) is derived from abraded soil, road dust (e.g., brake and tire dust), construction debris, or aggregation of smaller combustion particles, whereas fine (≤2.5 µm) and ultrafine (≤0.1 µm) PM is primarily formed during the combustion of fossil fuel products. PM size is directly linked to the potential for causing health problems. PM (<10 µm) generally passes through the throat and nose and enters the lungs, then can adversely affect different body organs, especially the heart and lungs. Although PM2.5 is a PM10 subset, the former contains only smaller particles, which have less mass but may be more respirable and hence more toxic. Diesel exhaust is a major source of fine particulates in urban areas, accounting for most airborne PM (up to 90%). Fine particles in diesel exhaust may enhance allergic and inflammatory responses to antigen challenges and may facilitate the development of new allergies31). Many epidemiologic studies consistently showed that PM can cause asthma and allergy in children14,16,25,26).
2. O3
O3 is not emitted directly into air because it is a secondary pollutant. In the presence of sunlight, it is created at ground-level by a chemical reaction between oxides of nitrogen and hydrocarbons. O3 may have harmful effects when formed in the earth's lower atmosphere, i.e., at ground-level. O3 is known to reach higher levels at higher temperatures. Therefore, global warming increases the concentration of ground-level O3. The harmful effects of O3 on allergic disease have been well studied, and it is well known that O3 exacerbates existing asthma and decreases lung function. Higher exposure to ambient O3 increases the risk of asthma and allergic rhinitis among children as well. In addition, it can cause allergic sensitization. Korean data also supported significant association of O3 with asthma, allergic rhinitis, and allergic sensitization17).
3. Nitrogen dioxide (NO2)
Automobile exhaust is the most significant source of outdoor NO2, a precursor to photochemical smog, and results in the production of O3 (NO2+ultraviolet sunlight+hydrocarbons=O3). Therefore, its major effects on health as an outdoor pollutant are probably mediated through the formation of O3. It can also induce an inflammatory response in the airways. Compared with its direct effects on the airways, NO2 may play a more prominent role as a sensitizing agent to inhaled allergens. Exposure to an ambient level of NO2 can enhance the allergic response after subsequent challenge with nonsymptomatic allergen dose32). It should be noted, however, that an independent role of NO2 cannot be clearly established because increased levels of ambient NO2 may be a marker for exposure to traffic emissions or other combustion-related pollution, causing high covariation between ambient NO2 and other pollutants.
4. Sulfur dioxide (SO2)
SO2 is a gas formed when fuel containing sulfur, such as coal and oil, is burned. Therefore, it is particularly abundant in industrial areas. Exposure (5 minutes) to inhaled SO2 induces rapid-onset bronchoconstriction (decreases in forced expiratory volume in 1 second or increases in airway resistance within 2 minutes of exposure) in both healthy and asthmatic subjects33). In response to SO2, asthmatic subjects experience increased symptoms and a greater decrease in pulmonary function at lower concentrations (0.25 ppm) compared with nonasthmatic subjects, who are often unresponsive at concentrations of less than 5 ppm33).
5. Possible mechanisms: oxidative stress and epigenetic mechanisms
Along with epidemiologic studies, increasing evidence from experimental studies has suggested that exposure to DEPs also has immunologic effects. Several in vivo and in vitro animal and human studies have strongly suggested that DEPs act as adjuvants and augment the allergic reaction34,35) (Fig. 2). Combined exposure to DEPs and antigens could switch the immune response toward immunoglobulin E36). However, the underlying molecular mechanisms of susceptibility and disease remain to be elucidated. A few potential mechanisms are briefly described below.
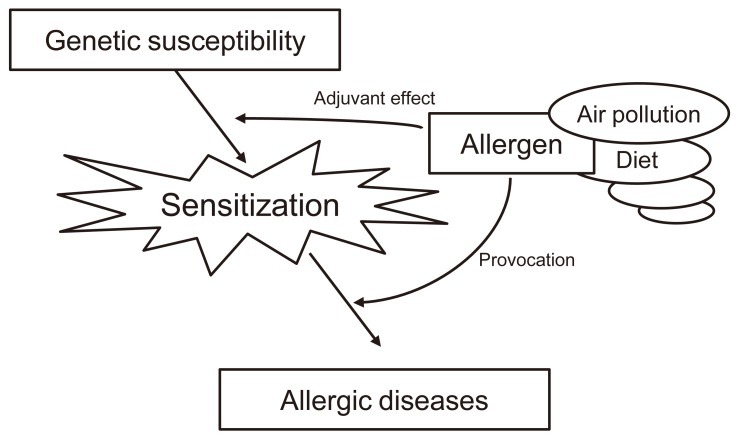
Ambient (outdoor) air pollution, such as diesel exhaust particulates, can act as adjuvants for allergic sensitization and augment the allergic reaction.
Many air pollutants exert their major effects by causing oxidative stress in cells and tissues that they contact. Gaseous pollutants (O3, SO2, and NO2) and particulate matter (PM and DEP) are known to form reactive oxygen species (ROS), such as superoxide anions, hydrogen peroxide, and hydroxyl radicals. Oxidative stress arises when ROS overwhelm antioxidant defenses. After this imbalance is reached, ROS readily react with proteins, lipids, and DNA, resulting in a number of pathological consequences37). A primary consequence of oxidative stress is lipid peroxidation, or the oxidative degeneration of lipids. If this process is not stopped, this reaction can permanently damage cell membranes, ultimately leading to cell death. Activation of signaling pathways is another way in which oxidant pollutants may cause pathological responses in the lung38). Air pollutants and ROS can activate mitogen-activated protein kinase signaling, which may ultimately promote inflammation. Therefore, dietary and genetic factors that influence the redox system may modulate the effects of air pollution on allergic diseases. A diet rich in antioxidants was found to be associated with a low prevalence of allergic disease39). The positive effects of antioxidant supplementation were predominantly found in children genetically susceptible to the effects of oxidants40). This is an example of gene and environmental interactions (Fig. 3).
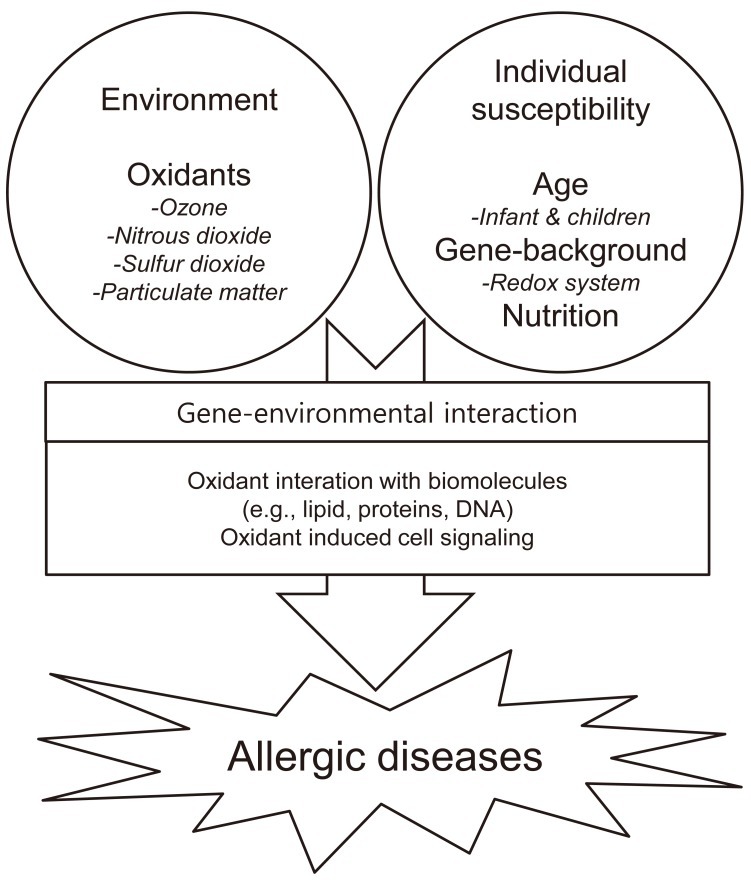
Flowchart demonstrating the interactions between gene and environmental factors, as well as the mechanisms of oxidative stress and susceptibility factors.
In the last several years, special attention has been given to the role of epigenetics in so-called environmental diseases such as allergies because epigenetic mechanisms control the expression of gene products, both basally and as a response to environmental challenge, without affecting the sequence of the DNA itself41). These mechanisms include structural regulation of chromatin structure, such as DNA methylation and histone modifications, and post-transcriptional gene regulation, such as microRNA-mediated repression of gene expression.
A recent animal study indicates that exposure to DEPs induces epigenetic changes. Concomitant exposure to ambient DEPs and antigen in mice alters the methylation of T helper genes42). Changes in methylation affect the differentiation of T helper cells, and therefore possibly also affect the risk of allergic sensitization and asthma. Therefore, epigenetic mechanisms, which mediate not only genetic and physiological responses to certain environmental insults, but also underlying susceptibility to environmental stressors, are likely to be targets for the prevention of allergy. More recently, changes in the expression of some microRNAs, small noncoding RNAs working as key regulators of gene expression, have been suggested to be critical for mediating biological, and ultimately physiological, responses to air pollutants43).
Prospective view: further studies and possible strategies for preventing and treating allergic diseases
Many epidemiologic studies showed that air pollution contributes to the development of allergic diseases and allergic sensitization14-17,21-26). These results have been supported by experimental studies, which revealed the immunological effects of air pollution and shed light on possible mechanisms to explain the harmful effects of air pollution34-36). Now there is an increasing need to evaluate the effects of air pollution on the development and long-term outcomes of different phenotypes of allergic disease, i.e., asthma and wheezing symptoms. In addition, further genetic association studies are required and may identify children at risk to air pollution. The understanding of how these mechanisms regulate gene-environment interactions to environmental exposures may lead us to new methods of controlling allergic diseases. For example, short-term randomized supplementation trials suggested that antioxidant vitamins and n-3 polyunsaturated fatty acids may protect against the acute effects of these pollutants, particularly in vulnerable subgroups44).
Environmental and genetic factors differ among different countries; therefore, national data are necessary for managing patients and establishing national policies for air quality. However, little Korean data are available45,46), and previous studies have not implemented validated regression models for assessing individual exposure levels; moreover, health outcomes have often been assessed using self-reported questionnaires rather than objective biomarkers. Along with the CHEER study described previously17,24), the COhort study for Childhood Origin of Asthma and allergic diseases (COCOA)47), a Korean birth cohort study conducted since 2008 (www.cdc.go.kr/phwr)48), may identify at-risk Korean children and give recommendations to the government on the implementation of effective air pollution policies.
Conclusions
The prevalence of allergic disease has increased worldwide, a phenomenon that can be largely attributed to environmental effects. Air pollution has been given special attention as a major cause of allergic diseases, particularly in children. Oxidative stress and epigenetic mechanisms are considered as possible mechanisms for developing allergic disease and sensitization. Further studies to identify children at risk and understand how these mechanisms regulate gene-environment interactions will lead us to develop new methods of controlling or preventing allergic diseases in children. Pediatricians need to understand the harmful effects of air pollution and integrate this knowledge into managing allergic diseases, including enhancement of patient education.
Acknowledgment
This study was supported by a grant (A092076) of the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea.