Clinical utilization of cord blood over human health: experience of stem cell transplantation and cell therapy using cord blood in Korea
Article information
Abstract
Cord blood (CB) has been used as an important and ethical source for hematopoietic stem cell transplantation (SCT) as well as cell therapy by manufacturing mesenchymal stem cell, induced pleuripotential stem cell or just isolating mononuclear cell from CB. Recently, the application of cell-based therapy using CB has expanded its clinical utility, particularly, by using autologous CB in children with refractory diseases. For these purposes, CB has been stored worldwide since mid-1990. In this review, I would like to briefly present the historical development of clinical uses of CB in the fields of SCT and cell therapy, particularly to review the experiences in Korea. Furthermore, I would touch the recent banking status of CB.
Introduction
The clinical use of cord blood (CB) has expanded into various areas of stem cell transplantation (SCT) such as treatment of malignant or nonmalignant hematologic diseases and inherited metabolic diseases1,2). In addition to being an important source for hematopoietic reconstitution, CB has been used experimentally and clinically to reconstitute impaired human tissues based on the studies reporting that the important sources for cell therapy, such as mesenchymal stem cell (MSC) or induced pleuripotential stem cell (iPS), could be isolated from CB3,4). The various mechanisms of hematopoietic reconstitution during SCT have been explored, and it has been found that intravenously infused CB could migrate to damaged BM microenvironment after the conditioning chemo-/radiotherapy and engraftment for hematopoietic reconstitution. In the field of cell therapy, administered MSC or iPS could be expected to differentiate to numerous tissue types with functional improvements. However, a lot of investigators believe that MSC would exert their immunomodulatory effects by regenerating damaged tissues, although the exact mechanism of tissue regeneration after cell therapy has not been found so far5,6,7). Because of this clinical applicability of CB, the storage of would-be wasted CB has been attempted and many CB banks have been established around the world.
SCT using CB
Since Broxmeyer8) had suggested that CB could be a source of transplantable hematopoietic stem cells (HSCs), a lot of experimental studies have been performed for clinical applications. The scientific findings revealed that HSCs in CB have an extensive proliferative capacity, which exceeds that of bone marrow (BM) HSC, and that the number of HSCs in a single CB collection was within the range of HSC numbers associated with successful bone marrow transplant (BMT). These studies led to the first SCT in a child with Fanconi anemia using the CB from a sibling and >30,000 CBSCTs (CBTs) have been performed so far1,9,10,11).
Over the last 25 years since the first successful CBT, a lot of progress has been made to overcome the limitations of CBT. The previous experiences of BMT showed that the infused cell dose was critical for engraftment after SCT. Therefore, the volume of BM to be collected should be adjusted according to the recipients' body weight. On the contrary, SCT using CB was impossible in relatively heavy patients when cell dose from a single collection of CB was insufficient. The first strategy to overcome the low cell contents in a single CB unit was to increase the infused stem cell dose using double unit of CB12) or ex vivo expanded CB13). Other efforts for enhancing the engraftment kinetics to overcome the limitations of low cell dose were intraosseous injection of CB14,15) and cotransplantation of third-party MSCs16).
Currently, double CBT has become the most popular method to overcome the limitations of cell dose. Increasing cell dose with cotransfusion of 2 partially human leukocyte antigen (HLA)-matched units revealed some advantages and disadvantages in outcomes. Compared to single CBT, double CBT was accompanied by a higher incidence of grade II acute graft-versus-host disease (GVHD). However, treatment-related mortality (TRM) or chronic GVHD were not higher but a higher graft-versus-leukemia effect was anticipated17,18,19,20,21,22). When 2 units of CB were transplanted, very interesting engraftment kinetics were revealed: early after double CBT (day +21) both CB units contributed to hematopoiesis in 40%-50% of patients, but by day +100 one unit predominated in the vast majority of the patients17,23). The unit predominance may be influenced by postthaw viability24), length of time interval between the infusion of the two CB units25) and ex vivo expansion26,27). The importance of T cells to establish chimerism and to ensure stem cell engraftment has been widely documented28,29,30,31,32,33).
Because donor selection is more important in unrelated CBT compared to BMT or peripheral blood SCT, several factors in addition to cell dose and HLA mismatch should be considered to select the best CB units: combined effect of HLA disparity and cell dose, HLA antibodies, and noninherited maternal antigen (NIMA). In HLA-mismatched CBT, the greater HLA-mismatch exists, the more total nucleated cell (TNC) dose is required. The 4/6 HLA-matched units to the recipient required a TNC >5.0×107/kg to achieve a similar TRM as 5/6 units with a TNC >2.5×107/kg34). Another important factor is the presence of HLA antibodies against the CB units, which has a negative prognostic impact in both single and double CBTs35,36,37). Two recent studies pointed out the importance of NIMA, demonstrating a survival advantage (5-year overall survival of 55% vs. 38%) by choosing CB units in which maternal typing of the CB donor showed a match of the noninherited maternal allele to the patient38,39).
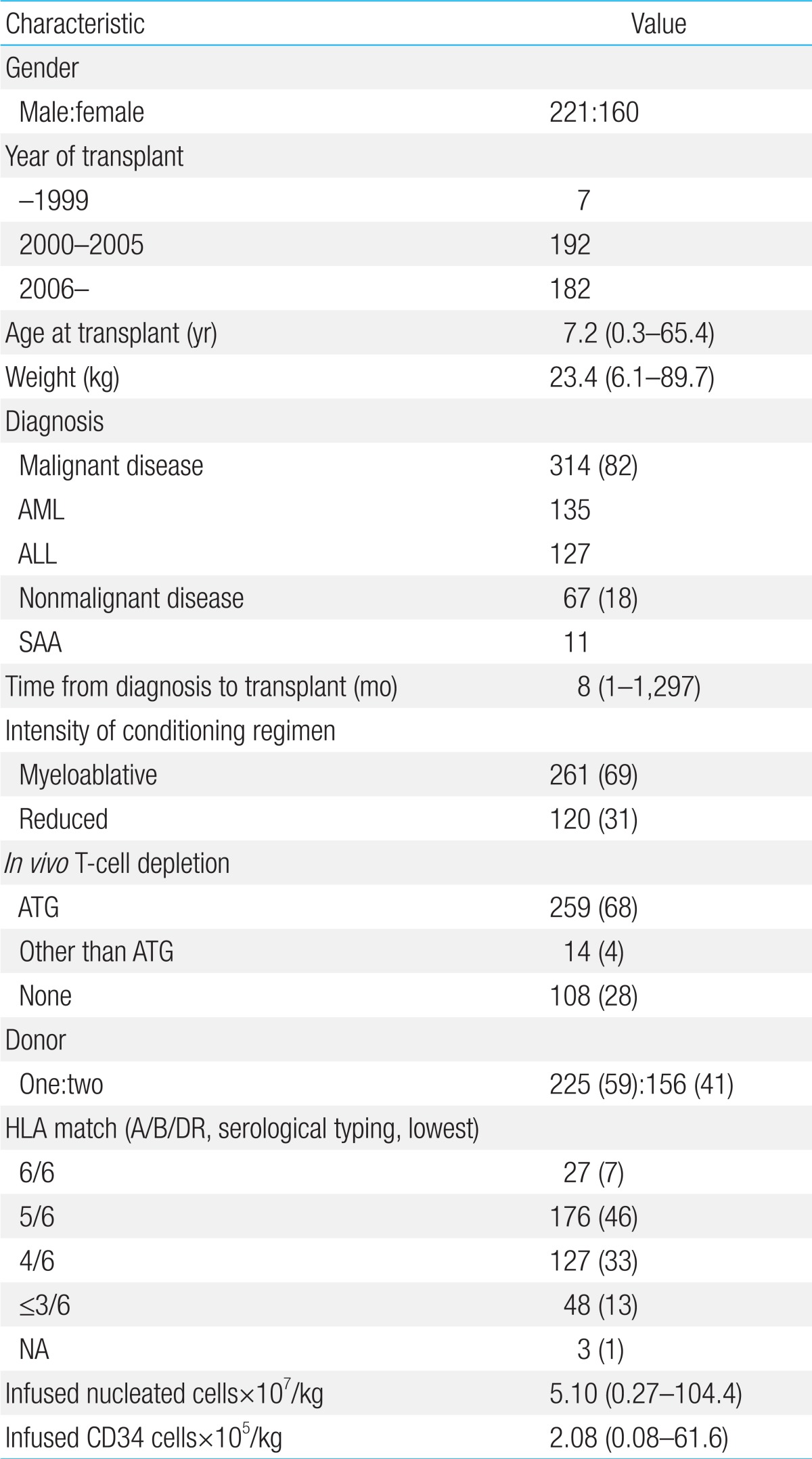
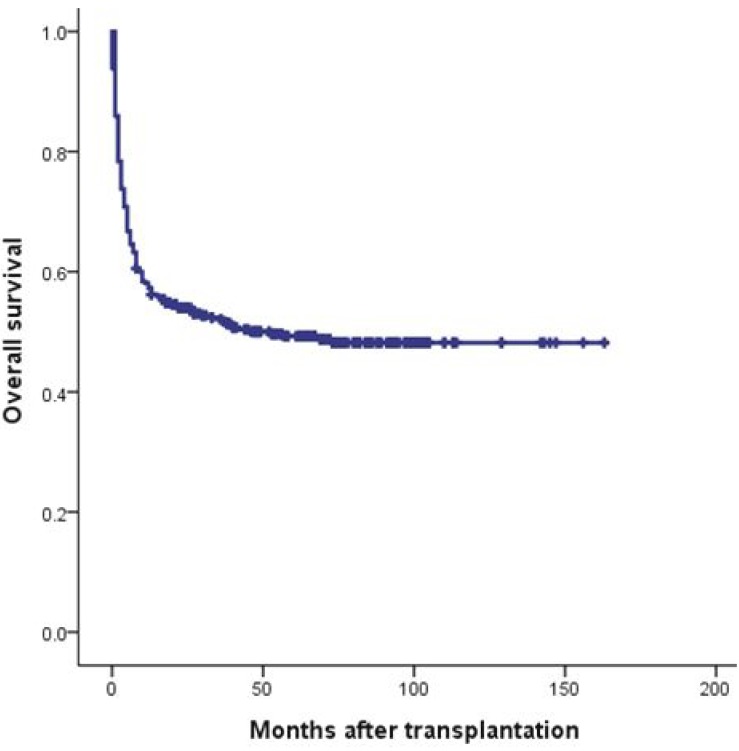
Since the first successful CBT in 199840) and double CBT in 200441), about 500 cases of CBT have been performed in Korea. Recently, CBT Working Party of The Korean Society of Hematology has performed a retrospective, multicenter study to reveal the clinical outcomes, including relevant risk factors of CBT, in Korea42). Data of 381 patients who received unrelated CBT were collected retrospectively from 19 medical centers in Korea between 1996 and 2011. Transplant characteristics were as follows (Table 1): median age, 7.2 years (range, 0.5-65.4 years); median weight, 23.4 kg (range, 6.1-89.7 kg); 58.0% male; 40.9% double-unit CBT; 83.1% hematologic malignant disease; and myeloablative regimen in 68.5%. The patients received a median of 2.08×105/kg CD34+ cells and a median of 5.10×107/kg nucleated cells. There were no significant differences between single- and double-unit CBT regarding the numbers of infused CD34+ cells and nucleated cells. Pre-engraftment syndrome (pES), a distinctive clinical syndrome which occurs during the engraftment process, developed in 102 patients (26.8%) at a median of 6.5 days. Median times for leukocyte and platelet engraftment were 18 and 44 days, respectively. Graft failure occurred in 20.5% of total patients. Factors associated with graft failure were nonmalignant disease (P<0.01), infused CD34+cells ≤1.0×105/kg (P<0.01), and absence of pES (P<0.01). Cumulative incidence (CI) of grade II-IV acute GVHD by day +100 and chronic GVHD at 1 year after transplantation were 40.25% and 20.9%, respectively. Cytomegalovirus (CMV) reactivation was found in 176 patients (46.2%) and 61 patients (16.0%) developed CMV disease. With a median follow-up of 74 months, 184 patients are alive with a predicted 5-year overall survival of 49.2% (Fig. 1) and event-free survival of 37.7% (Fig. 2). In multivariate analysis, adverse risk factors for survival included older age (P=0.002), salvage transplantation (P=0.04), CMV disease (P=0.03) and infused CD34+cells ≤1.0×105/kg (P=0.02). CI of TRM by 1 year after transplantation was 35.2%. Infection was the main cause of death with estimated infection-related mortality of 20.6%. Conclusively, the outcomes after CBT were comparable to those of other countries.
Cell therapy using CB
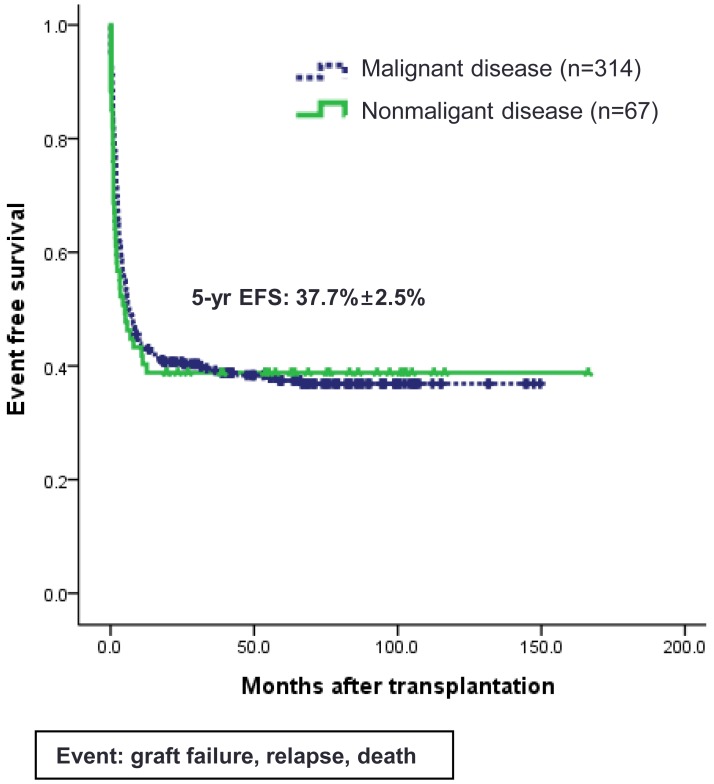
Since CB contains HSCs as well as a mixture of multipotent stem cells, CB has the ability to give rise to hematopoietic, epithelial, endothelial and neural tissues (Fig. 3). Recently, the application of cell-based therapy using CB has expanded its clinical utility, particularly, by manufacturing MSC and iPS. However, CB MNCs before any ex vivo manipulation could also be a good cellular source for tissue regeneration, because CB MNC contains immunomodulatory lymphocytes expressing various cytokines as well as progenitor cells including MSCs43). Some researchers also demonstrated that intralesional or intravenous administration of MNCs without any ex vivo manipulations could also have a potential role for tissue regeneration44,45). Based on the recent clinical experiences46,47,48,49), CB MNCs would be an ethically helpful therapeutic tool in a variety of diseases such as hemato-oncologic diseases, autoimmune diseases and diabetes, and neurologic diseases. In contrast to conventional SCT using CB, nonhematopoietic applications such as cardiovascular or neurological indications do not require permanent graft survival because the therapeutic activities of the CB are believed to be mediated in many cases by immunomodulatory mechanism, such as the secretion of neuroprotective, angiogenetic, and anti-inflammatory cytokines5,6,7). Although most clinical trials using BM- or CB-derived MSC have involved adult patients with degenerating neurologic diseases or spinal cord injuries50), nonhematologic therapeutic application using CB MNC for children have been performed to restore damaged tissue functions in patients with diabetes, neonatal hypoxic-ischemic encephalopathy, autism, and cerebral palsy (CP)46,47,48,49).
Cord blood contains hematopoietic stem cells as well as multipotent stem cells, such as mesenchymal stem cells, which have the ability to regenerate numerous tissue types. RBC, red blood cell; WBC, white blood cell.
A clinical trial using autologous CB for children with type 1 diabetes reported the enhanced blood glucose control and management, with retention of endogenous insulin production as assessed by stimulated C-peptide secretion, although another report failed to preserve C-peptide46). The mechanism of action is not yet known, but recent clinical trials suggest that infused CB may support both islet maintenance and regeneration as well as restoring the aberrant immune system51).
Kurtzberg et al.52) tried to use intravenous infusion of autologous CB to reveal the safety and the improvement of neurologic dysfunction in children with CP53), and they are performing a randomized controlled study as well. My colleagues also conducted a pilot study of the infusion of intravenous autologous CB in children with CP to assess the safety and feasibility of the procedure as well as its potential efficacy in countering neurological impairment. Twenty patients who received autologous CB infusion were evaluated. Infusion was generally well-tolerated, although 5 patients experienced temporary nausea, hemoglobinuria, or urticaria during the intravenous infusion. Diverse neurological domains improved in 5 patients (25%) as assessed with developmental evaluation tools and fractional anisotropy values in brain magnetic resonance imaging-diffusion tensor image. The neurologic improvement occurred significantly in patients with diplegia or hemiplegia rather than quadriplegia. We concluded that autologous CB infusion is safe and feasible, and has yielded potential benefits in children with CP49). Another group in Korea recently reported that the intravenous infusion of allogeneic CB MNCs has a therapeutic potential for CP54). They suggested that allogeneic CB MNC infusion could be a good source for amelioration of motor and cognitive dysfunction in children with CP, accompanied by structural and metabolic changes in the brain. The mechanism of action in these studies has not been explored yet, but many investigators believe that cytokines contained in CB may exert their neuroprotective, angiogenetic, and anti-inflammatory effects to improve neurologic functions. In conclusion, cellular therapy using CB MNC could be another good option for tissue regeneration because it is more safe and ethical compared to BM- or CB-derived MSC. Further studies are needed, particularly, in the field of non-hematologic application.
Storage of CB
1. CB collection and processing
CB is donated by healthy mothers after an uncomplicated pregnancy and after written informed consent. In Korea, the guidelines and criteria for collection and processing of donated CB are presented in the "Cord Blood Management and Research Act" (CB Act) which has taken effect since 201155).
Collected CB is processed to isolate MNCs with automatic or semiautomatic process using centrifugation. The red blood cells and plasma are discarded into the satellite bag, leaving a mean volume of about 20-25 mL of MNCs and plasma in the bag. The whole process is performed in a closed system with the use of a sterile connecting device. Dimethyl sulfoxide/dextran (10% dimethyl sulfoxide in 5% dextran) is added as a cryoprotectant and the product is cryopreserved by controlled-rate freezing in a 25 mL double-compartment cryopreservation bag. Cryopreserved CB units are stored under liquid nitrogen until using them. Recently, Broxmeyer et al.4) reported the efficiency of long-term cryopreservation of CB, showing that the recovery of functional hematopoietic progenitor cells cryopreserved as MNCs for upto 23.5 years was comparable to prefreeze values of the same CB units.
2. CB banks
There are two types of CB banks around the world: government or community-funded public banks and for-profit private banks. Public CB banks store donated CB for public access, and are analogous to volunteer BM donor registries. In contrast, private CB banks commercially preserve a child's CB for personal or family use under the agreement with contractor. A global CB banks network, NetCord, is being operated to use donated CB for public purposes, and more than 300,000 units are currently registered as of 2013. In Korea, we established the networking system of 7 public CB banks in October 2006 and more than 30,000 units are currently registered as of July 2012. Since the enactment of CB Act in 2011, all CB data are affiliated with the Korean Hematopoietic Stem Cell Donor Registry which is nationally coordinated. On the other hand, the need for private banking business is controversial, but millions of units were estimated to have been preserved around the globe already and more than 300,000 units are preserved in Korea4).
The clinical applicability of CB has been widened and is expected to be a good source for unrelated SCT as well as cell therapy. However, there is still much to be resolved to increase the utilization of banked CB, especially for physicians who have to select optimal unrelated stem cell donors or try cell therapy using autologous CB units. The utilization rate of private CB is extremely low (0.04%-0.0005%)56). In addition, the utilization rate of banked public CB is approximately 3%-4% in World Marrow Donor Association, 3% in National Marrow Donor Program (until 2010), 29% in Japan (until 2012), and 1.3% in Korea (until 2012)56). Therefore, the strategy to promote the utilization of banked public and private CB should be considered.
Conclusions
Since CB is used as an important source for saving life and regenerating human tissue, would-be wasted CB has to be controlled strictly. Basic and clinical researches to improve the clinical outcomes of SCT using CB would be warranted. In addition, experimental and clinical trials using CB MNC or CB-derived MSC for cell therapy would be encouraged, because a lot of CB is stored in private CB banks around the world and their use is very safe and ethical.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This study was supported by a grant of Korea Healthcare Technology R&D Project (A101712), Ministry for Health & Welfare, Republic of Korea.