Article Contents
| Korean J Pediatr > Volume 57(3); 2014 |
|
Abstract
Cord blood (CB) has been used as an important and ethical source for hematopoietic stem cell transplantation (SCT) as well as cell therapy by manufacturing mesenchymal stem cell, induced pleuripotential stem cell or just isolating mononuclear cell from CB. Recently, the application of cell-based therapy using CB has expanded its clinical utility, particularly, by using autologous CB in children with refractory diseases. For these purposes, CB has been stored worldwide since mid-1990. In this review, I would like to briefly present the historical development of clinical uses of CB in the fields of SCT and cell therapy, particularly to review the experiences in Korea. Furthermore, I would touch the recent banking status of CB.
The clinical use of cord blood (CB) has expanded into various areas of stem cell transplantation (SCT) such as treatment of malignant or nonmalignant hematologic diseases and inherited metabolic diseases1,2). In addition to being an important source for hematopoietic reconstitution, CB has been used experimentally and clinically to reconstitute impaired human tissues based on the studies reporting that the important sources for cell therapy, such as mesenchymal stem cell (MSC) or induced pleuripotential stem cell (iPS), could be isolated from CB3,4). The various mechanisms of hematopoietic reconstitution during SCT have been explored, and it has been found that intravenously infused CB could migrate to damaged BM microenvironment after the conditioning chemo-/radiotherapy and engraftment for hematopoietic reconstitution. In the field of cell therapy, administered MSC or iPS could be expected to differentiate to numerous tissue types with functional improvements. However, a lot of investigators believe that MSC would exert their immunomodulatory effects by regenerating damaged tissues, although the exact mechanism of tissue regeneration after cell therapy has not been found so far5,6,7). Because of this clinical applicability of CB, the storage of would-be wasted CB has been attempted and many CB banks have been established around the world.
Since Broxmeyer8) had suggested that CB could be a source of transplantable hematopoietic stem cells (HSCs), a lot of experimental studies have been performed for clinical applications. The scientific findings revealed that HSCs in CB have an extensive proliferative capacity, which exceeds that of bone marrow (BM) HSC, and that the number of HSCs in a single CB collection was within the range of HSC numbers associated with successful bone marrow transplant (BMT). These studies led to the first SCT in a child with Fanconi anemia using the CB from a sibling and >30,000 CBSCTs (CBTs) have been performed so far1,9,10,11).
Over the last 25 years since the first successful CBT, a lot of progress has been made to overcome the limitations of CBT. The previous experiences of BMT showed that the infused cell dose was critical for engraftment after SCT. Therefore, the volume of BM to be collected should be adjusted according to the recipients' body weight. On the contrary, SCT using CB was impossible in relatively heavy patients when cell dose from a single collection of CB was insufficient. The first strategy to overcome the low cell contents in a single CB unit was to increase the infused stem cell dose using double unit of CB12) or ex vivo expanded CB13). Other efforts for enhancing the engraftment kinetics to overcome the limitations of low cell dose were intraosseous injection of CB14,15) and cotransplantation of third-party MSCs16).
Currently, double CBT has become the most popular method to overcome the limitations of cell dose. Increasing cell dose with cotransfusion of 2 partially human leukocyte antigen (HLA)-matched units revealed some advantages and disadvantages in outcomes. Compared to single CBT, double CBT was accompanied by a higher incidence of grade II acute graft-versus-host disease (GVHD). However, treatment-related mortality (TRM) or chronic GVHD were not higher but a higher graft-versus-leukemia effect was anticipated17,18,19,20,21,22). When 2 units of CB were transplanted, very interesting engraftment kinetics were revealed: early after double CBT (day +21) both CB units contributed to hematopoiesis in 40%-50% of patients, but by day +100 one unit predominated in the vast majority of the patients17,23). The unit predominance may be influenced by postthaw viability24), length of time interval between the infusion of the two CB units25) and ex vivo expansion26,27). The importance of T cells to establish chimerism and to ensure stem cell engraftment has been widely documented28,29,30,31,32,33).
Because donor selection is more important in unrelated CBT compared to BMT or peripheral blood SCT, several factors in addition to cell dose and HLA mismatch should be considered to select the best CB units: combined effect of HLA disparity and cell dose, HLA antibodies, and noninherited maternal antigen (NIMA). In HLA-mismatched CBT, the greater HLA-mismatch exists, the more total nucleated cell (TNC) dose is required. The 4/6 HLA-matched units to the recipient required a TNC >5.0×107/kg to achieve a similar TRM as 5/6 units with a TNC >2.5×107/kg34). Another important factor is the presence of HLA antibodies against the CB units, which has a negative prognostic impact in both single and double CBTs35,36,37). Two recent studies pointed out the importance of NIMA, demonstrating a survival advantage (5-year overall survival of 55% vs. 38%) by choosing CB units in which maternal typing of the CB donor showed a match of the noninherited maternal allele to the patient38,39).
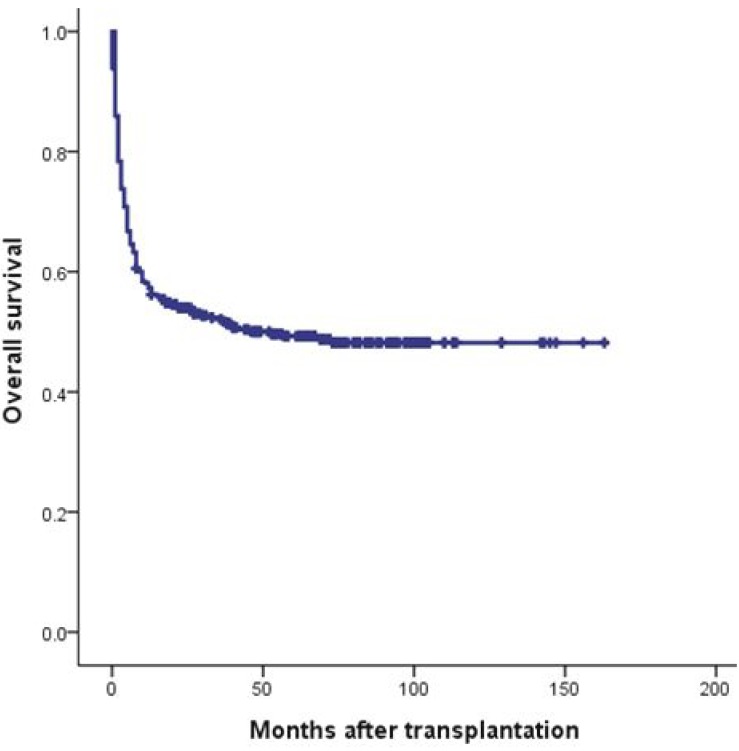
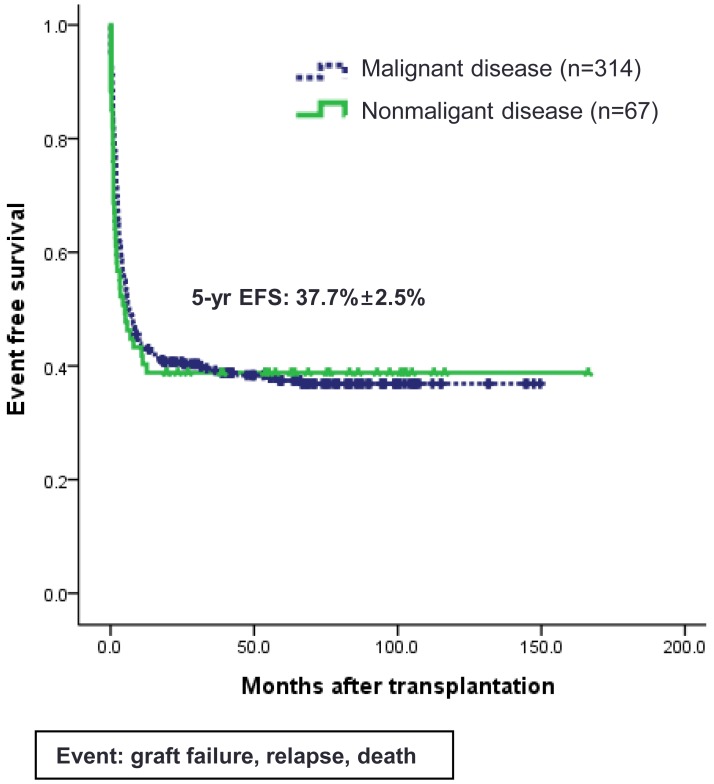
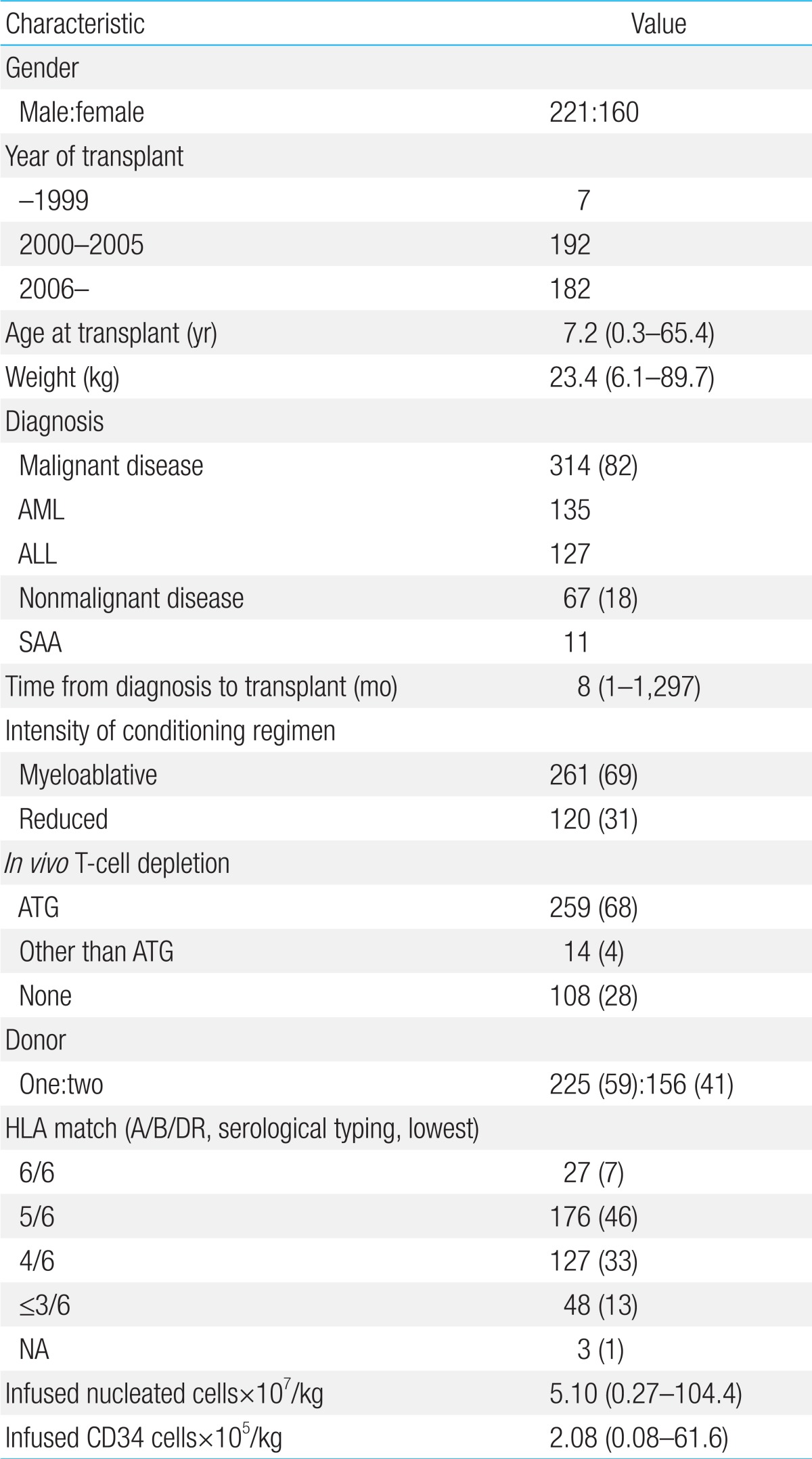
Since the first successful CBT in 199840) and double CBT in 200441), about 500 cases of CBT have been performed in Korea. Recently, CBT Working Party of The Korean Society of Hematology has performed a retrospective, multicenter study to reveal the clinical outcomes, including relevant risk factors of CBT, in Korea42). Data of 381 patients who received unrelated CBT were collected retrospectively from 19 medical centers in Korea between 1996 and 2011. Transplant characteristics were as follows (Table 1): median age, 7.2 years (range, 0.5-65.4 years); median weight, 23.4 kg (range, 6.1-89.7 kg); 58.0% male; 40.9% double-unit CBT; 83.1% hematologic malignant disease; and myeloablative regimen in 68.5%. The patients received a median of 2.08×105/kg CD34+ cells and a median of 5.10×107/kg nucleated cells. There were no significant differences between single- and double-unit CBT regarding the numbers of infused CD34+ cells and nucleated cells. Pre-engraftment syndrome (pES), a distinctive clinical syndrome which occurs during the engraftment process, developed in 102 patients (26.8%) at a median of 6.5 days. Median times for leukocyte and platelet engraftment were 18 and 44 days, respectively. Graft failure occurred in 20.5% of total patients. Factors associated with graft failure were nonmalignant disease (P<0.01), infused CD34+cells ≤1.0×105/kg (P<0.01), and absence of pES (P<0.01). Cumulative incidence (CI) of grade II-IV acute GVHD by day +100 and chronic GVHD at 1 year after transplantation were 40.25% and 20.9%, respectively. Cytomegalovirus (CMV) reactivation was found in 176 patients (46.2%) and 61 patients (16.0%) developed CMV disease. With a median follow-up of 74 months, 184 patients are alive with a predicted 5-year overall survival of 49.2% (Fig. 1) and event-free survival of 37.7% (Fig. 2). In multivariate analysis, adverse risk factors for survival included older age (P=0.002), salvage transplantation (P=0.04), CMV disease (P=0.03) and infused CD34+cells ≤1.0×105/kg (P=0.02). CI of TRM by 1 year after transplantation was 35.2%. Infection was the main cause of death with estimated infection-related mortality of 20.6%. Conclusively, the outcomes after CBT were comparable to those of other countries.
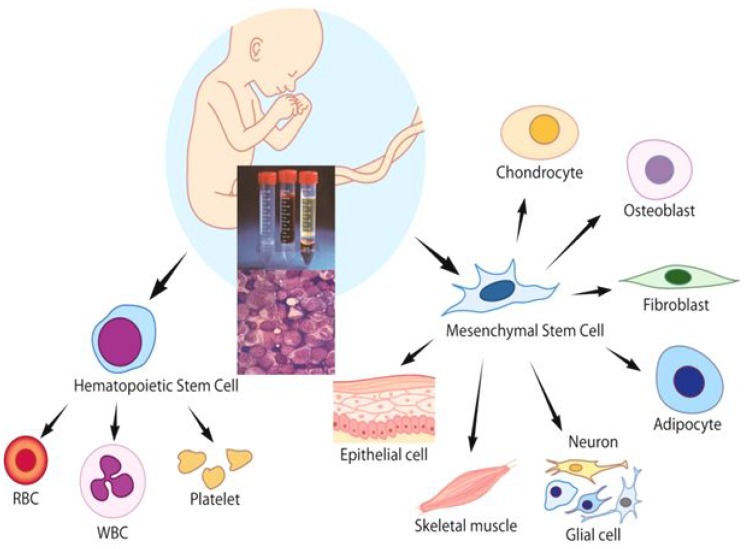
Since CB contains HSCs as well as a mixture of multipotent stem cells, CB has the ability to give rise to hematopoietic, epithelial, endothelial and neural tissues (Fig. 3). Recently, the application of cell-based therapy using CB has expanded its clinical utility, particularly, by manufacturing MSC and iPS. However, CB MNCs before any ex vivo manipulation could also be a good cellular source for tissue regeneration, because CB MNC contains immunomodulatory lymphocytes expressing various cytokines as well as progenitor cells including MSCs43). Some researchers also demonstrated that intralesional or intravenous administration of MNCs without any ex vivo manipulations could also have a potential role for tissue regeneration44,45). Based on the recent clinical experiences46,47,48,49), CB MNCs would be an ethically helpful therapeutic tool in a variety of diseases such as hemato-oncologic diseases, autoimmune diseases and diabetes, and neurologic diseases. In contrast to conventional SCT using CB, nonhematopoietic applications such as cardiovascular or neurological indications do not require permanent graft survival because the therapeutic activities of the CB are believed to be mediated in many cases by immunomodulatory mechanism, such as the secretion of neuroprotective, angiogenetic, and anti-inflammatory cytokines5,6,7). Although most clinical trials using BM- or CB-derived MSC have involved adult patients with degenerating neurologic diseases or spinal cord injuries50), nonhematologic therapeutic application using CB MNC for children have been performed to restore damaged tissue functions in patients with diabetes, neonatal hypoxic-ischemic encephalopathy, autism, and cerebral palsy (CP)46,47,48,49).
A clinical trial using autologous CB for children with type 1 diabetes reported the enhanced blood glucose control and management, with retention of endogenous insulin production as assessed by stimulated C-peptide secretion, although another report failed to preserve C-peptide46). The mechanism of action is not yet known, but recent clinical trials suggest that infused CB may support both islet maintenance and regeneration as well as restoring the aberrant immune system51).
Kurtzberg et al.52) tried to use intravenous infusion of autologous CB to reveal the safety and the improvement of neurologic dysfunction in children with CP53), and they are performing a randomized controlled study as well. My colleagues also conducted a pilot study of the infusion of intravenous autologous CB in children with CP to assess the safety and feasibility of the procedure as well as its potential efficacy in countering neurological impairment. Twenty patients who received autologous CB infusion were evaluated. Infusion was generally well-tolerated, although 5 patients experienced temporary nausea, hemoglobinuria, or urticaria during the intravenous infusion. Diverse neurological domains improved in 5 patients (25%) as assessed with developmental evaluation tools and fractional anisotropy values in brain magnetic resonance imaging-diffusion tensor image. The neurologic improvement occurred significantly in patients with diplegia or hemiplegia rather than quadriplegia. We concluded that autologous CB infusion is safe and feasible, and has yielded potential benefits in children with CP49). Another group in Korea recently reported that the intravenous infusion of allogeneic CB MNCs has a therapeutic potential for CP54). They suggested that allogeneic CB MNC infusion could be a good source for amelioration of motor and cognitive dysfunction in children with CP, accompanied by structural and metabolic changes in the brain. The mechanism of action in these studies has not been explored yet, but many investigators believe that cytokines contained in CB may exert their neuroprotective, angiogenetic, and anti-inflammatory effects to improve neurologic functions. In conclusion, cellular therapy using CB MNC could be another good option for tissue regeneration because it is more safe and ethical compared to BM- or CB-derived MSC. Further studies are needed, particularly, in the field of non-hematologic application.
CB is donated by healthy mothers after an uncomplicated pregnancy and after written informed consent. In Korea, the guidelines and criteria for collection and processing of donated CB are presented in the "Cord Blood Management and Research Act" (CB Act) which has taken effect since 201155).
Collected CB is processed to isolate MNCs with automatic or semiautomatic process using centrifugation. The red blood cells and plasma are discarded into the satellite bag, leaving a mean volume of about 20-25 mL of MNCs and plasma in the bag. The whole process is performed in a closed system with the use of a sterile connecting device. Dimethyl sulfoxide/dextran (10% dimethyl sulfoxide in 5% dextran) is added as a cryoprotectant and the product is cryopreserved by controlled-rate freezing in a 25 mL double-compartment cryopreservation bag. Cryopreserved CB units are stored under liquid nitrogen until using them. Recently, Broxmeyer et al.4) reported the efficiency of long-term cryopreservation of CB, showing that the recovery of functional hematopoietic progenitor cells cryopreserved as MNCs for upto 23.5 years was comparable to prefreeze values of the same CB units.
There are two types of CB banks around the world: government or community-funded public banks and for-profit private banks. Public CB banks store donated CB for public access, and are analogous to volunteer BM donor registries. In contrast, private CB banks commercially preserve a child's CB for personal or family use under the agreement with contractor. A global CB banks network, NetCord, is being operated to use donated CB for public purposes, and more than 300,000 units are currently registered as of 2013. In Korea, we established the networking system of 7 public CB banks in October 2006 and more than 30,000 units are currently registered as of July 2012. Since the enactment of CB Act in 2011, all CB data are affiliated with the Korean Hematopoietic Stem Cell Donor Registry which is nationally coordinated. On the other hand, the need for private banking business is controversial, but millions of units were estimated to have been preserved around the globe already and more than 300,000 units are preserved in Korea4).
The clinical applicability of CB has been widened and is expected to be a good source for unrelated SCT as well as cell therapy. However, there is still much to be resolved to increase the utilization of banked CB, especially for physicians who have to select optimal unrelated stem cell donors or try cell therapy using autologous CB units. The utilization rate of private CB is extremely low (0.04%-0.0005%)56). In addition, the utilization rate of banked public CB is approximately 3%-4% in World Marrow Donor Association, 3% in National Marrow Donor Program (until 2010), 29% in Japan (until 2012), and 1.3% in Korea (until 2012)56). Therefore, the strategy to promote the utilization of banked public and private CB should be considered.
Since CB is used as an important source for saving life and regenerating human tissue, would-be wasted CB has to be controlled strictly. Basic and clinical researches to improve the clinical outcomes of SCT using CB would be warranted. In addition, experimental and clinical trials using CB MNC or CB-derived MSC for cell therapy would be encouraged, because a lot of CB is stored in private CB banks around the world and their use is very safe and ethical.
Acknowledgments
This study was supported by a grant of Korea Healthcare Technology R&D Project (A101712), Ministry for Health & Welfare, Republic of Korea.
References
1. Wagner JE, Kernan NA, Steinbuch M, Broxmeyer HE, Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet 1995;346:214–219.


2. Boelens JJ. Trends in haematopoietic cell transplantation for inborn errors of metabolism. J Inherit Metab Dis 2006;29:413–420.


3. Zhang X, Hirai M, Cantero S, Ciubotariu R, Dobrila L, Hirsh A, et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem 2011;112:1206–1218.


4. Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood 2011;117:4773–4777.



5. Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han ZC. Human umbilical cord blood cells express neurotrophic factors. Neurosci Lett 2005;380:322–325.


6. Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Willing AE, et al. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci 2008;9:22



7. Xiao J, Nan Z, Motooka Y, Low WC. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev 2005;14:722–733.


8. Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A 1989;86:3828–3832.



9. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 1989;321:1174–1178.


10. Wagner JE, Broxmeyer HE, Byrd RL, Zehnbauer B, Schmeckpeper B, Shah N, et al. Transplantation of umbilical cord blood after myeloablative therapy: analysis of engraftment. Blood 1992;79:1874–1881.


11. Kohli-Kumar M, Shahidi NT, Broxmeyer HE, Masterson M, Delaat C, Sambrano J, et al. Haemopoietic stem/progenitor cell transplant in Fanconi anaemia using HLA-matched sibling umbilical cord blood cells. Br J Haematol 1993;85:419–422.


12. Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med 2001;344:1870–1871.

13. Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant 2002;8:368–376.


14. Frassoni F, Gualandi F, Podesta M, Raiola AM, Ibatici A, Piaggio G, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol 2008;9:831–839.


15. Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, McKenna D, Chong SY, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant 2009;43:935–940.



16. Gonzalo-Daganzo R, Regidor C, Martin-Donaire T, Rico MA, Bautista G, Krsnik I, et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy 2009;11:278–288.


17. Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 2007;110:3064–3070.



18. MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood 2009;113:2410–2415.



19. Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 2009;27:256–263.


20. Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 2009;114:4293–4299.



21. Goldstein G, Elhasid R, Bielorai B, Shimoni A, Yerushalmi R, Kassis I, et al. Adults requiring cord blood transplants but have insufficient cell doses from a single cord blood unit can receive two units with successful engraftment kinetics similar to those of children receiving a single unit. Leuk Lymphoma 2011;52:635–641.


22. Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010;116:4693–4699.



23. Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant 2007;13:82–89.



24. Scaradavou A, Smith KM, Hawke R, Schaible A, Abboud M, Kernan NA, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant 2010;16:500–508.


25. Haspel RL, Kao G, Yeap BY, Cutler C, Soiffer RJ, Alyea EP, et al. Preinfusion variables predict the predominant unit in the setting of reduced-intensity double cord blood transplantation. Bone Marrow Transplant 2008;41:523–529.


26. Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 2010;16:232–236.



27. De Lima M, Robinson S, McMannis JD, Alousi AM, Saliba RM, Munsell M, et al. Mesenchymal stem cell based cord blood expansion leads to rapid engraftment of platelets and neutrophils. Blood 2010;116:164

28. Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 2001;98:3192–3204.


29. Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood 1991;78:2120–2130.


30. Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005;105:1343–1347.


31. Gutman JA, Turtle CJ, Manley TJ, Heimfeld S, Bernstein ID, Riddell SR, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood 2010;115:757–765.



32. Kanda J, Rizzieri DA, Gasparetto C, Long GD, Chute JP, Sullivan KM, et al. Adult dual umbilical cord blood transplantation using myeloablative total body irradiation (1350 cGy) and fludarabine conditioning. Biol Blood Marrow Transplant 2011;17:867–874.


33. Eldjerou LK, Chaudhury S, Baisre-de Leon A, He M, Arcila ME, Heller G, et al. An in vivo model of double-unit cord blood transplantation that correlates with clinical engraftment. Blood 2010;116:3999–4006.



34. Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood 2010;115:1843–1849.



35. Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood 2011;118:6691–6697.



36. Ruggeri A, Rocha V, Masson E, Labopin M, Cunha R, Absi L, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Société Francophone d'Histocompatibilité et d'Immunogénétique (SFHI) and Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) analysis. Haematologica 2013;98:1154–1160.



37. Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood 2010;116:2839–2846.


38. Rocha V, Spellman S, Zhang MJ, Ruggeri A, Purtill D, Brady C, et al. Effect of HLA-matching recipients to donor noninherited maternal antigens on outcomes after mismatched umbilical cord blood transplantation for hematologic malignancy. Biol Blood Marrow Transplant 2012;18:1890–1896.


39. van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci U S A 2009;106:19952–19957.



40. Lee YH, Cho NC, Je KH, Han H, Han JY, Kim JS, et al. Successful sibling cord blood stem cell transplantation for relapsed acute mixed lineage leukemia. Korean J Hematol 1999;34:471–476.
41. Kang HJ, Yoo KH, Lee JW, Kim H, Lee SH, Sung KW, et al. Double umbilical cord blood transplantation for children and adolescents. Ann Hematol 2010;89:1035–1044.



42. Park M, Lee YH, Lee SH, Yoo KH, Sung KW, Koo HH, et al. Current status of unrelated cord blood transplantation in Korea. Korean J Hematol 2012;47(Suppl 1): 63
43. Takahashi N, Uehara R, Kobayashi M, Yada Y, Koike Y, Kawamata R, et al. Cytokine profiles of seventeen cytokines, growth factors and chemokines in cord blood and its relation to perinatal clinical findings. Cytokine 2010;49:331–337.


44. Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg 2009;138:646–653.


45. Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001;32:2682–2688.


46. Haller MJ, Wasserfall CH, McGrail KM, Cintron M, Brusko TM, Wingard JR, et al. Autologous umbilical cord blood transfusion in very young children with type 1 diabetes. Diabetes Care 2009;32:2041–2046.



47. Liao Y, Cotten M, Tan S, Kurtzberg J, Cairo MS. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Bone Marrow Transplant 2013;48:890–900.


48. Lv YT, Zhang Y, Liu M, Qiuwaxi JN, Ashwood P, Cho SC, et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med 2013;11:196



49. Lee YH, Choi KV, Moon JH, Jun HJ, Kang HR, Oh SI, et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med 2012;10:58



50. Momin EN, Mohyeldin A, Zaidi HA, Vela G, Quinones-Hinojosa A. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther 2010;5:326–344.


51. Haller MJ, Wasserfall CH, Hulme MA, Cintron M, Brusko TM, McGrail KM, et al. Autologous umbilical cord blood transfusion in young children with type 1 diabetes fails to preserve C-peptide. Diabetes Care 2011;34:2567–2569.



52. ClinicalTrials.gov. Kurtzberg JA randomized study of autologous umbilical cord blood reinfusion in children with cerebral palsy [Internet]. Bethesda: ClinicalTrials.gov, cited 2013 Sep 17. Available from: http://clinicaltrials.gov/show/NCT01147653.
53. Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S, et al. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion 2010;50:1980–1987.


54. Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells 2013;31:581–591.


Fig. 3
Cord blood contains hematopoietic stem cells as well as multipotent stem cells, such as mesenchymal stem cells, which have the ability to regenerate numerous tissue types. RBC, red blood cell; WBC, white blood cell.



 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


