Excellent treatment outcomes in children younger than 18 months with stage 4 MYCN nonamplified neuroblastoma
Article information
Abstract
Purpose
Although the prognosis is generally good in patients with intermediate-risk neuroblastoma, no consensus has been reached on the ideal treatment regimen. This study analyzed treatment outcomes and toxicities in patients younger than 18 months with stage 4 MYCN nonamplified neuroblastoma.
Methods
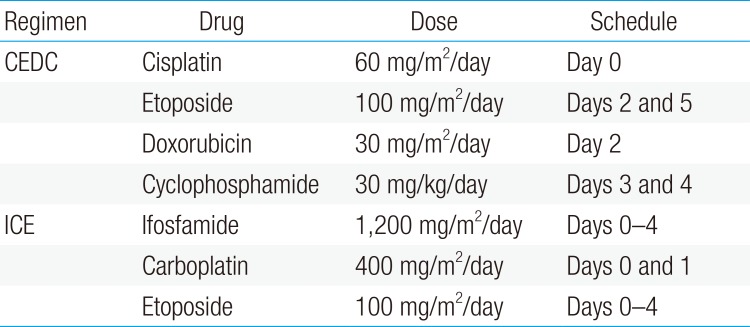
We retrospectively analyzed 20 patients younger than 18 months newly diagnosed with stage 4 MYCN nonamplified neuroblastoma between January 2009 and December 2015. Patients received 9 cycles of chemotherapy and surgery, with or without local radiotherapy, followed by 12 cycles of differentiation therapy with 13-cis-retinoic acid. Chemotherapy consisted of alternating cycles of cisplatin, etoposide, doxorubicin, and cyclophosphamide (CEDC) and ifosfamide, carboplatin, and etoposide (ICE) regimens.
Results
The most common primary tumor site was the abdomen (85%), and the most common metastatic sites were the lymph nodes (65%), followed by the bones (60%), liver (55%), skin (45%), and bone marrow (25%). At the end of induction therapy, 14 patients (70%) achieved complete response, with 1 achieving very good partial response, 4 achieving partial response, and 1 showing mixed response. Nine patients (45%) received local radiotherapy. At a median follow-up of 47 months (range, 17–91 months), none of these patients experienced relapse, progression, or secondary malignancy, or died. Three years after chemotherapy completion, none of the patients had experienced grade ≥3 late adverse effects.
Conclusion
Patients younger than 18 months with stage 4 MYCN nonamplified neuroblastoma showed excellent outcomes, without significant late adverse effects, when treated with alternating cycles of CEDC and ICE, followed by surgery and differentiation therapy.
Introduction
Neuroblastoma is the most common extracranial solid tumor in children, constituting 6%–10% of all childhood cancers.1) The course of this disease varies, depending on the biologic features of the tumor.23) Patients with neuroblastoma are classified into risk groups by age, stage, MYCN amplification status, DNA index, and histology. Treatments stratified by risk classification have improved survival outcomes in patients with neuroblastoma.456)
Although most pediatric solid tumors with metastasis at diagnosis are considered high-risk,78) metastatic neuroblastoma can be classified as intermediate-risk, depending on other prognostic factors. The Children's Oncology Group (COG) has classified patients with stage 4 neuroblastoma as being at intermediate-risk, if they are younger than 12 months of age without MYCN amplification, or 12–18 months of age with a hyperdiploid DNA index, favorable tumor histology, and no MYCN amplification.9) In contrast, the International Neuroblastoma Risk Group (INRG) classified patients with stage 4 neuroblastoma as being at intermediate-risk, if they were younger than 18 months of age with a diploid DNA index and without MYCN amplification.10)
Previous trials have reported overall survival (OS) rates >80% among patients with intermediate-risk neuroblastoma.4611) However, these trials differed in treatment modalities, including chemotherapeutic agents and doses, as well as in application of local radiation therapy. Our institution has classified patients younger than 18 months old with stage 4 MYCN nonamplified neuroblastoma as intermediate-risk. In our hospital, these patients has been treated with 9 cycles of chemotherapy and surgery, with or without local radiotherapy, followed by differentiation therapy with 13-cis-retinoic acid (CRA). This study analyzed the treatment outcomes and toxicities of this treatment protocol.
Materials and methods
1. Patients
From January 2009 to December 2015, 20 consecutive patients younger than 18 months of age were newly diagnosed with stage 4 MYCN nonamplified neuroblastoma at the Samsung Medical Center. All patients were treated with chemotherapy and surgery, with or without local radiation therapy, followed by differentiation therapy. Patient records were reviewed retrospectively. The study was approved by the Institutional Review Board at Samsung Medical Center, Seoul, Korea (approval number: 2017-02-031), which waived the requirement for informed consent.
2. Assessment of disease status
Neuroblastoma was diagnosed by histological examination of the tumor specimens. The extent of the disease was evaluated using computed tomography or magnetic resonance imaging, a technetium-99 bone scan, bilateral bone marrow aspirate and biopsy specimens, and an iodine-123-metaiodobenzylguanidine (MIBG) scan. Patients were staged according to criteria of the International Neuroblastoma Staging System.12) MYCN copy number was determined using fluorescence in situ hybridization. Tumor pathology was determined by using the international neuroblastoma pathology classification (INPC).13) Routine evaluation at diagnosis included measurement of serum lactate dehydrogenase (LDH), ferritin, neuron-specific enolase (NSE), and vanillylmandelic acid (VMA) concentrations.14)
3. Induction therapy
All patients received 9 cycles of chemotherapy consisting of alternating cisplatin, etoposide, doxorubicin, and cyclophosphamide (CEDC) and ifosfamide, carboplatin, and etoposide (ICE) regimens (Table 1). An excisional biopsy of the primary tumor was obtained at diagnosis if the tumor was deemed resectable; otherwise, incisional or percutaneous needle biopsy was performed, and definitive surgery was deferred until after 6 cycles of chemotherapy. After surgery, all patients received 3 cycles of postoperative chemotherapy.
4. Local radiotherapy
From January 2009 to May 2012, all patients received local radiotherapy, regardless of residual tumor after induction therapy. From June 2012 to December 2014, local radiotherapy was administered only to patients with gross residual tumor after induction therapy. Beginning in January 2015, local radiotherapy was administered only to patients with gross residual tumor and residual MIBG uptake after induction therapy. Local radiotherapy was started 4 weeks after the completion of induction chemotherapy, at doses of 15 Gy for patients without gross residual tumor and 23.4–25.2 Gy for patients with gross residual tumors. Radiotherapy was not administered to metastatic sites.
5. Differentiation therapy
One month after the completion of induction therapy, patients were treated with CRA to differentiate any possible residual tumor cells. Treatment consisted of 12 cycles of CRA (125 mg/m2/day) for 14 days every 4 weeks.
6. Definitions and response criteria
An event was defined as the occurrence of relapse, progression, a secondary malignancy, or death. Treatment response was evaluated by international response criteria.12) Briefly, complete response (CR) was defined as no identifiable tumor with normal catecholamine levels. Very good partial response (VGPR) was defined as 90%–99% reduction in primary tumor size with normal catecholamine levels, without evidence of metastatic disease, including resolution of MIBG uptake, and with or without residual 99Tc-bone changes. Partial response (PR) was defined as >50% reduction in the sizes of the primary tumor and any metastatic sites. A mixed response (MR) was defined as a >50% reduction of any measurable lesion, with a <50% reduction in other lesions, or a <25% increase in any lesion. Stable disease (SD) defined as a response <50%, and progressive disease (PD) was defined as a >25% increase in any preexisting tumor or the appearance of a new lesion.
7. Evaluation of late adverse effects
Endocrine, auditory, ophthalmologic, respiratory, renal, cardiac, and cognitive functions were evaluated 3 years after completion of induction therapy to detect possible late adverse effects. Toxicities were determined according to the common terminology criteria for adverse events (version 4.03) of the US National Cancer Institute. Neurocognitive function was evaluated using the Korean-Wechsler Preschool and Primary Scale of Intelligence.
Results
1. Patient characteristics
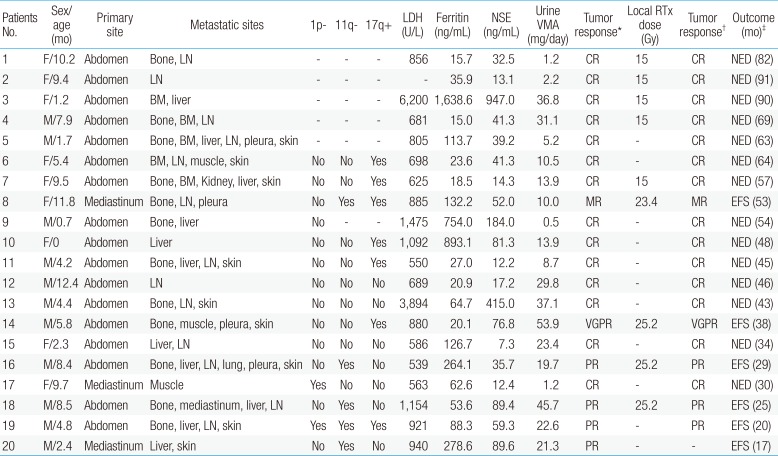
The median age of the 20 patients at diagnosis was 5.6 months (range, 0–12.4 months). The most common primary site was the abdomen (85%), and the most common metastatic site was the lymph nodes (65%), followed by the bones (60%), liver (55%), skin (45%), and bone marrow (25%). Four patients (20%) had unfavorable INPC tumor pathology. Cytogenetic analysis was possible in 15 patients, with 1p deletions detected in 2 patients, 11q deletions in 5, and 17q gains in 7. The median LDH, ferritin, and NSE concentrations were 856 U/L (range, 539–6,200 U/L), 63.7 ng/mL (range, 15.0–1,638.6 ng/mL), and 41.3 ng/mL (range, 7.3–947.0 ng/mL), respectively, and the median 24-hour urine VMA was 16.8 mg/day (range, 0.5–53.9 mg/day). Other detailed patients characteristics are listed in Table 2.
2. Treatment and outcomes
Of the 20 patients, 19 (95%) completed 9 cycles of chemotherapy, whereas 1 patient (patient No. 5) received only 8 cycles of chemotherapy due to renal tubulopathy. At the end of induction therapy, 14 patients (70%) achieved CR, 1 (5%) achieved VGPR, 4 (20%) achieved PR, and 1 (5%) achieved MR, with no patient showing SD or PD. Between January 2009 and May 2012, 5 patients (patients Nos. 1, 2, 3, 4, and 7) received local radiotherapy, whereas 2 did not due to renal tubulopathy (patient No. 5) and parental refusal (patient No. 6). From June 2012 to December 2014, 2 patients (patients Nos. 8 and 14) with residual tumors after induction therapy received local radiotherapy. From January 2015 to the present, 2 patients patients Nos. 16 and 18) with residual tumors and MIBG uptake received local radiotherapy. Two patients (patients Nos. 19 and 20) with residual tumors but no MIBG uptakes at the end of induction therapy and who did not receive local radiotherapy remain event-free after follow-up periods of 20 and 17 months, respectively. Fourteen patients who achieved CR at the end of induction therapy had maintained CR at the end of differentiation therapy, and 6 patients also remained event-free. At a median follow-up of 47 months (range, 17–91 months) from diagnosis, all of the patients remained event-free.
3. Late adverse effects
Late adverse effects were evaluated in 12 patients 3 years after the end of induction therapy (Table 3). The most frequent adverse effect was hearing loss (50%), but none of these patients required a hearing aid or experienced cochlear impairment. No patient experienced a grade ≥3 late adverse effect. Their median height 3 years after the end of induction therapy was 0.11 standard deviations above the mean height with respect to patient age. Fig. 1 shows individual heights from the time of diagnosis to the end of follow-up. The median value for full-scale intelligence quotient was 95 (range, 87–115).
Discussion
We retrospectively reviewed the clinical and biologic characteristics and treatment outcomes in patients younger than 18 months of age with stage 4 neuroblastoma without MYCN amplification. Previous trials have shown favorable survival rates in patients with intermediate-risk neuroblastoma. For example, the 3-year event-free survival rate of infants with stage 4 MYNC nonamplified neuroblastoma was 93%±4%,15) and survival outcomes were reported to be significantly higher in infants than in children (83% vs. 45%, P<0.001).16) In our study, 75% of patients achieved CR or VGPR at the end of induction therapy, with no patients experiencing relapse, progression, secondary malignancy, or death during follow-up. These results indicated that patients aged <18 months with stage 4 MYCN nonamplified neuroblastoma had excellent treatment outcomes.
Risk-based treatment stratification based on prognostic factors was developed both to improve treatment outcomes and minimize toxicities in patients with neuroblastoma.456) Age at diagnosis has been the one of the most important prognostic factors for neuroblastoma. Traditionally, 12 months of age has been used as the cutoff in risk stratification, but recent studies have revealed that patients with neuroblastoma stage 4 MYCN nonamplified aged 12–18 months showed favorable outcomes compared with patients aged >18 months.1116) Thereafter, recent classifications have used not only 12 but also 18 months as an age cutoff.910) However, guidelines differ in stratifying risk. For example, the COG has classified patients aged <18 months with stage 4 MYCN nonamplified tumors as being at intermediate- or high-risk, depending on tumor cell DNA index and tumor histology.9) In contrast, the INRG has classified patients aged <18 months with metastatic MYCN nonamplified tumors as being at intermediate- or low-risk, depending on tumor cell DNA index.10) We classified patients aged <18 months with stage 4 MYCN nonamplified neuroblastoma as being at intermediate-risk, regardless of DNA index. Although no patient in our study experienced disease progression, had we incorporated DNA index into our risk stratification, some of our patients may have instead been classified as high-risk, which may have affected treatment and follow-up strategies. In contrast, incorporation of other biological tumor factors, such as DNA index, into risk stratification may reduce treatment toxicity for patients at lower-risk. As we did not experience any late high-grade toxicities, we believe that our stratification strategy was valid in this cohort, but this should be considered in future studies.
Reduced treatment intensity has shown satisfactory outcomes in patients of intermediate-risk neuroblastoma with favorable prognostic factors.4617) For example, 2 trials of reduced intensity treatment in 169 infants with stage 4 neuroblastoma without MYCN amplification yielded 2-year OS rates of 97.6% and 99.3%.6) Another study, in which patients with intermediate-risk neuroblastoma received either 4 or 8 cycles of chemotherapy (A3961 protocol) depending on DNA index, histopathologic features, or treatment response, showed a the 3-year OS rate of the entire group of 96%±1%.17) The drug doses and outcomes in studies of stage 4 MYNC nonamplified neuroblastoma are shown in Table 4. Although our patients were treated with higher cumulative chemotherapy doses than patients in these other studies, none of our patients experienced grade ≥3 late adverse effects 3 years after induction therapy. However, some of our patients experienced grades 1–2 late adverse effects, suggesting the need to reduce the intensity of treatment with intermediate-risk neuroblastoma and favorable prognostic factors.
Clinical trials have differed in their use of local radiotherapy to treat patients with intermediate-risk neuroblastoma. For example, in the Children's Cancer Group 3881 trial, local radiotherapy was administered if gross residual tumor remained after chemotherapy and surgery. In the COG A3961 trial, however, local radiotherapy was administered after chemotherapy and surgery only to patients with PD, relapsed tumors, or residual tumors with unfavorable biological characteristics.17) Other studies have omitted local radiotherapy altogether for patients with intermediate-risk neuroblastoma.46) Beginning in January 2015, none of our patients with residual tumors without MIBG uptake after completion of induction therapy received local radiotherapy. Although none of these patients experienced events during the follow-up period, the small number of patients and the relatively short follow-up in our study prevent our ability to conclusively determine the need for local radiotherapy in patients with residual lesions without MIBG uptakes. Larger trials with longer follow-up period are needed to assess the role of local radiotherapy in treating these lesions.
The role of radiotherapy in treating neuroblastoma metastases has not been completely determined. In the current study, 1 patient (patient No. 18) with MIBG uptake at metastatic sites at the end of induction therapy did not undergo local radiotherapy to these metastatic sites, and there was no MIBG uptake at these sites after differentiation therapy. A previous study reported that 8 of 10 infants with neuroblastoma and lymph node metastasis achieved locoregional control without radiotherapy to the metastatic sites.18) Moreover, survival rates did not differ significantly in 37 patients with high-risk neuroblastoma who did and did not receive radiotherapy to metastatic sites,19) further suggesting that radiotherapy to metastatic sites may be unnecessary for patients with neuroblastoma.
Although our sample size was small and the follow-up was short, the results of the present study showed that patients aged <18 months with stage 4 MYCN nonamplified neuroblastoma had excellent treatment outcomes without significant late adverse effects. Additional studies are needed to determine the best methods to reduce treatment intensity and duration without jeopardizing survival.
Acknowledgments
This study was supported by a grant from the Korean Society of Pediatric Hematology-Oncology 2012.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.