Increasing incidence of inflammatory bowel disease in children and adolescents: significance of environmental factors
Article information
Abstract
Inflammatory bowel disease (IBD) is a chronic relapsing immune-mediated disease of the intestinal tract. Although its prevalence is reportedly lower in Asia than in Western countries, the rapid increase in the incidence of IBD has drawn attention to its etiology, including genetic susceptibility and environmental factors. Specifically, recent studies concerning dietary treatments and intestinal microbiota suggest that these factors may interact with the immune system, and the imbalance of this relationship may lead to immune dysregulation in IBD. Changes in diet or alterations in the composition of the intestinal microbiota may be associated with the increasing incidence of IBD in Asia. Here, we aim to review recent studies on the role of diet and intestinal microbiota in IBD pathogenesis and the results of the investigations performed to modulate these factors.
Introduction
Inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC), is an immune-mediated chronic relapsing inflammatory condition that mainly affects the gastrointestinal tract.
The incidence and prevalence of IBD is increasing globally. Although reportedly higher in Western countries, a similar trend is now being observed in Asia as well. The prevalences of CD and UC in Canada in 2008 were 255.2 per 100,000 (95% confidence interval [CI], 252.4–258.0) and 259.7 per 100,000 (95% CI, 256.9–262.5), respectively, while the prevalences of CD and UC in the commercially-insured in the commercially-insured adult population in United States during 2008–2009 were 241.3 (95% CI, 238.1–244.5) and 263.0 (95% CI, 259.7–266.4) per 100,000, respectively [1,2]. In European studies, prevalences as high as those in North America have been reported [3]. Unlike in Western countries, epidemiologic data for IBD in Asia are relatively lacking, but the prevalence of IBD in these areas is thought to be lower. In a Japanese study, the prevalences of CD and UC in 2005 were 21.2 (95% CI, 20.8–21.7) and 63.6 (95% CI, 62.8–64.4) per 100,000, respectively [4]. In South Korea, the adjusted prevalences of CD and UC per 100,000 in 2005 were 11.24 (95% CI, 9.29–13.18) and 30.87 (95% CI, 27.47–34.27), respectively [5]. However, according to Korean cohort studies, the mean annual incidence seems to have increased by more than 100-fold for both CD and UC in the past 20 years [5].
Increasing occurrences are also being seen in pediatric patients. From 1994 to 2009, the incidence of IBD in children increased from 9.4 per 100,000 (95% CI, 8.2–10.8) to 13.2 per 100,000 (95% CI, 11.9–14.6) (P<0.0001) [6].
Various factors seem to contribute to the increasing global incidence of IBD, including an increased awareness of the disease, more developed disease surveillance systems, improved accessibility to hospitals and health care providers, and lifestyle changes toward a Westernized environment [7]. Although genetic factors appear to play an important role in the pathogenesis of IBD as shown in Swedish cohort studies on identical twins, environmental changes over the past few decades have been among the important factors increasing the incidence of IBD in Asia [3,8,9]. This is supported by the higher incidence of illness among immigrants who migrate from countries with a low incidence to Western countries [10].
The environmental changes and subsequent alterations to the intestinal microbiota are considered important in the increased prevalence of IBD in Asia. Therapeutic approaches involving these factors demonstrating efficacy in recent studies also support this proposition [11-17]. In this review, we will discuss the environmental, nutritional, and microbial factors that contribute to the pathogenesis of IBD as well as therapeutic approaches to modulate these factors.
Diet as an environmental factor
Dietary patterns and nutritional factors are considered important environmental factors in the etiology of IBD, and the Western diet has long been suspected of contributing to the development of IBD [10]. This diet is characterized by high levels of fats and refined sugars and low levels of fiber and vegetables. Several studies, although many were animal studies, have shown that the Western diet is associated with increased levels of proinflammatory cytokines, modulated intestinal permeability, and altered composition of the intestinal microbiota that promote chronic inflammation in the gut [18-21].
Sakamoto et al. [22] found a positive association between sugar consumption and CD risk (odds ratio [OR], 2.83; 95% CI, 1.38–5.83) and UC (OR, 2.86; 95% CI, 1.24–6.57). This supports views on the detrimental effects of refined carbohydrates and sweetened beverages in the Western diet on IBD. On the contrary, complex carbohydrates and fiber-rich vegetables and fruits are thought to be beneficial [23]. Haskey et al. showed that a high protein intake, especially from animal protein, resulted in a 3.3-time increased risk of IBD, suggesting that a diet high in animal protein was a major risk factor [23]. With respect to the intake of dietary fats, a high n-3 polyunsaturated fatty acid (PUFA) to n-6 PUFA ratio is reportedly inversely associated with the risk of IBD [23-25].
Several studies have shown the influence of certain components contained in food on epithelial cell permeability. For example, Söderholm et al. [26,27] found that sodium caprate, a medium-chain fatty acid in dairy products, increased the intestinal per me ability in the ilea of rats and humans, more noticeably in those with CD. Lammers et al. [28] proved that gliadin, the toxic component of gluten that initiates the inflammatory response in celiac disease binds to C-X-C motif chemokine receptor 3 in the small intestine epithelium to increase zonulin release in a myeloid-differentiating primary-response 88-dependent manner and subsequently increases intestinal permeability. Increased intestinal perme ability is associated with a defective mucosal barrier, which exposes the luminal bacteria and their products to the mucosa. An influx of the luminal contents to the intestinal mucosa triggers immune cell activation and cytokine production and secretion [29].
Aside from the nutritional food components, food additives such as detergents and emulsifiers may be associated with defective barrier function [30]. Chassaing et al. [31] documented in an animal study that chronic exposure to carboxymethylcellulose, a widely used cellulose derivative used as a viscosity modifier in a variety of dairy products, sauces, and sausages, increased bacterial adherence to the intestinal epithelium, particularly proinflammatory microbiota. Another food emulsifier common ly used in processed food, polysorbate-80, increased the translocation of Escherichia coli across M cells and Peyer’s patches in patients with CD [32].
Intestinal microbiota and IBD
The human microbiota consists of about 1,014 diverse microbes, primarily in the colon [33]. The gut microbiota affects human health by performing many roles in metabolite synthe sis, barrier function, and immune responses [34]. There is an interaction between the gut microbiota and the host immune system; when this balanced relationship is disrupted, gut microbial composition changes occur that consequently aggravate permeability dysfunction [35]. This, in turn, further aggravates alteration of the microbial composition, i.e., dysbiosis, to which a chronic inflammatory response occurs in the host immune system.
The intestinal microbiota is also known to have an important role in the pathogenesis of IBD [36]. Many studies have shown lower microbial diversity and a higher microbial dysbiosis index in IBD patients compared to healthy controls. Moreover, a long-term follow-up study of fecal microbiota in patients with IBD showed that the microbial profiles of IBD patients are different from those of healthy controls, and high volatility was observed in IBD patients [37].
Some specific changes in the intestinal microbiota of IBD patients have been identified, such as decreases in Roseburia hominis and Faecalibacterium prausnitzii, butyrate-producing species [38]. There are reports of an increased amount of Enterobacteriaceae and a reduced number of Clostridium clusters IV and XVIa during disease-associated inflammation in newly diagnosed CD patients [39]. Increases in Escherichia coli, Fusobacterium, and Proteus as well as decreases in Firmicutes such as Faecalibacterium prausnitzii have also been reported [3].
In newly diagnosed pediatric IBD patients, an increased amount of Proteobacteria and a decrease in Faecalibacterium prausnitzii in the intestinal microbiota seem to be associated with complicated disease phenotypes and a subsequent need for biologic therapy or surgery [40].
Diet and microbiota
Colonization of the gut begins at birth, and the gut microbiota become more stable and develop adult-like complexity during the first year of life [41]. Various environmental factors, such as diet, are known to contribute to this phenomenon, which begins very early in life. A higher proportion of Bifidobacteria was found in breastfed than formula-fed infants [42]. As previously mentioned, dietary patterns are associated with the pathogenesis of IBD and are related to the intestinal microbiota. In a mouse model, mice fed a high-fat diet demonstrated dysbiosis characterized by an increase in Proteobacteria and decrease in Firmicutes, similar to that observed in CD [43]. A high-fat diet results in the accumulation of secondary bile acids, which in turn can inhibit the growth of the Bacteroidetes and Firmicutes phyla, a common dysbiotic feature found in CD [44].
These varied evidences suggest that the dietary pattern influences the composition of the intestinal microbiota; through this indirect mechanism, diet can determine subsequent intestinal inflammation (Fig. 1).

Relationship between diet, intestinal microbiota, and inflammatory bowel disease (IBD). Genetic susceptibility, diet, and microbial composition contribute to the incidence of inflammatory bowel disease. Inflammatory bowel disease itself and its severity are responsible for the microbial composition; conversely, dysbiosis is also thought to affect inflammatory bowel disease.
Environmental modification as treatment
1. Dietary modification
Exclusive enteral nutrition (EEN) is the only evidence-based dietary treatment for IBD. EEN involves a completely liquid diet without any normal dietary components for a certain duration. It is used as a therapeutic method to induce remission in pediatric patients with active CD [45].
According to a meta-analysis, EEN is as effective as corticosteroids at inducing remission in pediatric CD patients. This is true for newly diagnosed CD and relapsed cases (OR, 0.76; 95% CI, 0.29–1.98). Furthermore, EEN was more effective than corticosteroids with respect to mucosal healing (OR, 4.5; 95% CI, 1.64–12.32) [46]. Based on these results, the European Crohn’s and Colitis Organization and the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) consensus guidelines on the medical management of pediatric CD recommended EEN as the first-line therapy to induce remission in children with active luminal CD [47]. Although its mechanisms are not yet fully understood, there is some evidence that EEN reinforces the epithelial barrier; accordingly, bacterial invasion into the mucosa is prevented. Moreover, strengthening the barrier can restore intestinal microbial dysbiosis and affect the dysregulated immune system by decreasing pro-inflammatory cytokines as well as the local action of anti-inflammatory cytokines. In this way, EEN is thought to improve inflammation in CD [48].
Despite the safety and efficacy of EEN for inducing remission in pediatric CD patients, intolerance of or poor adherence to is the main hindrance to implementing this therapeutic method [49]. A recent randomized clinical trial by Levine et al. [11] showed that the combination of the Crohn disease exclusion diet (CDED) and partial enteral nutrition (PEN) induced sustained remission at week 12 compared to EEN (75.6% and 45.1%, respectively, P=0.01). Moreover, significantly higher tolerance was noted in patients who received CDED with PEN than those treated with EEN (97.5% and 73.7% respectively, P=0.002).
Table 1 shows various attempts to develop a new dietary treatment method for IBD to target the modulation of intestinal microbiota or the immune system itself [11,50-60]. However, there is insufficient evidence to propose a recommendation. A recent position paper on behalf of the Porto Inflammatory Bowel Disease Group of the ESPGHAN also stated that “elimination or restrictive diet in children/adolescents with IBD should not be recommended unless potential benefits outweigh potential risks of the diet [61].”
2. Use of probiotics and prebiotics in the treatment of IBD
Ever since intestinal microorganisms were known to attribute to the pathogenesis of IBD, efforts have been made to restore the altered microbial composition. The administration of probiotics and prebiotics as IBD treatment has obtained inconsistent results [21].
Probiotics are defined as live microorganisms that are beneficial to the host’s health when ingested in adequate amounts. Derived from commensal microbiota, they mimic the homeostatic effect of intestinal microbiota in the healthy condition. They affect the mucosal immune system by balancing pro- and anti-inflammatory components, alter the microbial composition by inhibiting pathogenic bacteria, and enhance barrier function [62-65].
Although many probiotics demonstrated limited efficacy in UC, the probiotic cocktail VSL#3 and Escherichia coli Nissle 1917 reduced active inflammation in many studies [66-69]. They were associated with increased mucosal regulatory T cells, reduced pro-inflammatory cytokines, increased microbial diversity, and restored barrier integrity. However, they showed no statistically significant impact for treating CD [70,71]. In addition, several microbes such as Faecalibacterium prausnitzii and Bacteroides fragilis have been studied for their therapeutic poten tial as they seem to reduce colitis severity in many mouse models [72-74].
As shown above, some taxa seem to be associated with the disease course of IBD by interacting with the immune system [38-40]. However, the changes of specific microbiota that increase the risk of IBD are not completely clear. Therefore, it is important to manipulate specific microbiota and enhance the overall environment of beneficial microorganisms. Moreover, the fact that dietary modification affects the disease course of IBD also suggests the significance of prebiotics as a nutrient source for the microbiota.
Prebiotics are nondigestible food components fermented by microbiota that benefit the host. By providing essential food to the microbiota, prebiotics can modify the gut microbial com position and subsequent metabolites. For instance, oligosaccharides and fibers induce the proliferation of short-chain fatty acid-producing bacteria, such as the Bacteroides genus, to improve colitis severity in IBD [75,76]. Many in vitro and animal studies also confirmed that prebiotics helped alleviate colitis severity by altering the microbial composition, production of short-chain fatty acids such as butyrate, and balances in pro- and anti-inflammatory cytokines [77-80].
Several human studies have confirmed the efficacy of prebiotics in IBD patients, but the results are controversial. Lindsay et al. showed a reduced disease activity index and increased mucosal Bifidobacteria when CD patients were provided with 15 g of fructooligosaccharides (FOS) [81]. However, a randomized trial conducted by Benjamin et al. [82] showed no significant improvement in disease activity in a population that received FOS, although it demonstrated an increase in interleukin (IL)-10-producing dendritic cells (DCs) and decrease in IL-6-producing DCs. Other than FOS, germinated barley foodstuff, Ispaghula husk, and a combination of oligofructose and inulin demonstrated the potential to induce remission or reduce in flammation in UC patients, while inulin showed its efficacy in pouchitis patients as well [83-86].
As describe above, probiotics and prebiotics are thought to play distinct roles in immune system regulation. Although they are considered feasible armamentaria in the modulation of intestinal microbiota and possible treatment options for IBD, many clinical trials on this subject are tremendously heterogeneous in study design, mode of administration, and used probiotics or prebiotics to lead to a firm conclusion. Therefore, further well-designed studies of larger populations are needed to determine which probiotics or prebiotics effectively treat IBD.
Intestinal microbiota modification as a treatment using fecal microbiota transplantation
Fecal microbiota transplantation (FMT) is another approach to modulating the intestinal microbiota. FMT is considered the most direct method to manipulate the intestinal microbiota profile, and there is much ongoing research regarding FMT as a potential treatment for IBD.
As the first case of FMT in pseudomembranous colitis was reported several decades ago, FMT has been well studied and proven as an effective and safe treatment for refractory or recurrent Clostridium difficile infection [87]. This has stimulated research in FMT for other diseases related to dysbiosis, including IBD.
Although there are no available randomized controlled trials (RCTs) of CD patients, 6 uncontrolled cohort studies were reported with controversial results [12]. Vermeire et al. [13] reported a 0% remission rate among patients with moderate to severe CD, whereas Cui et al. [14] reported a 76.7% clinical remission rate after 4 weeks of FMT. Although a meta-analysis of the 6 pooled cohort studies showed a clinical remission rate of 52%, RCTs with a larger number of patients are needed to ensure the efficacy of FMT in CD patients [88].
In contrast to CD, larger RCTs have been performed in UC patients. A clinical remission rate of 24%–50% was reported in an FMT group; in 3 of 4 studies, remission rates were significantly higher in the FMT group than in the control group [15-17,88]. A meta-analysis of 4 RCTs also showed a significant benefit in clinical remission (pooled OR, 2.89; 95% CI, 1.36–6.13; P=0.006) [88]. Subsequently, the American Gastroenterological Association stated the efficacy of FMT for inducing remission in mild to moderate UC [89].
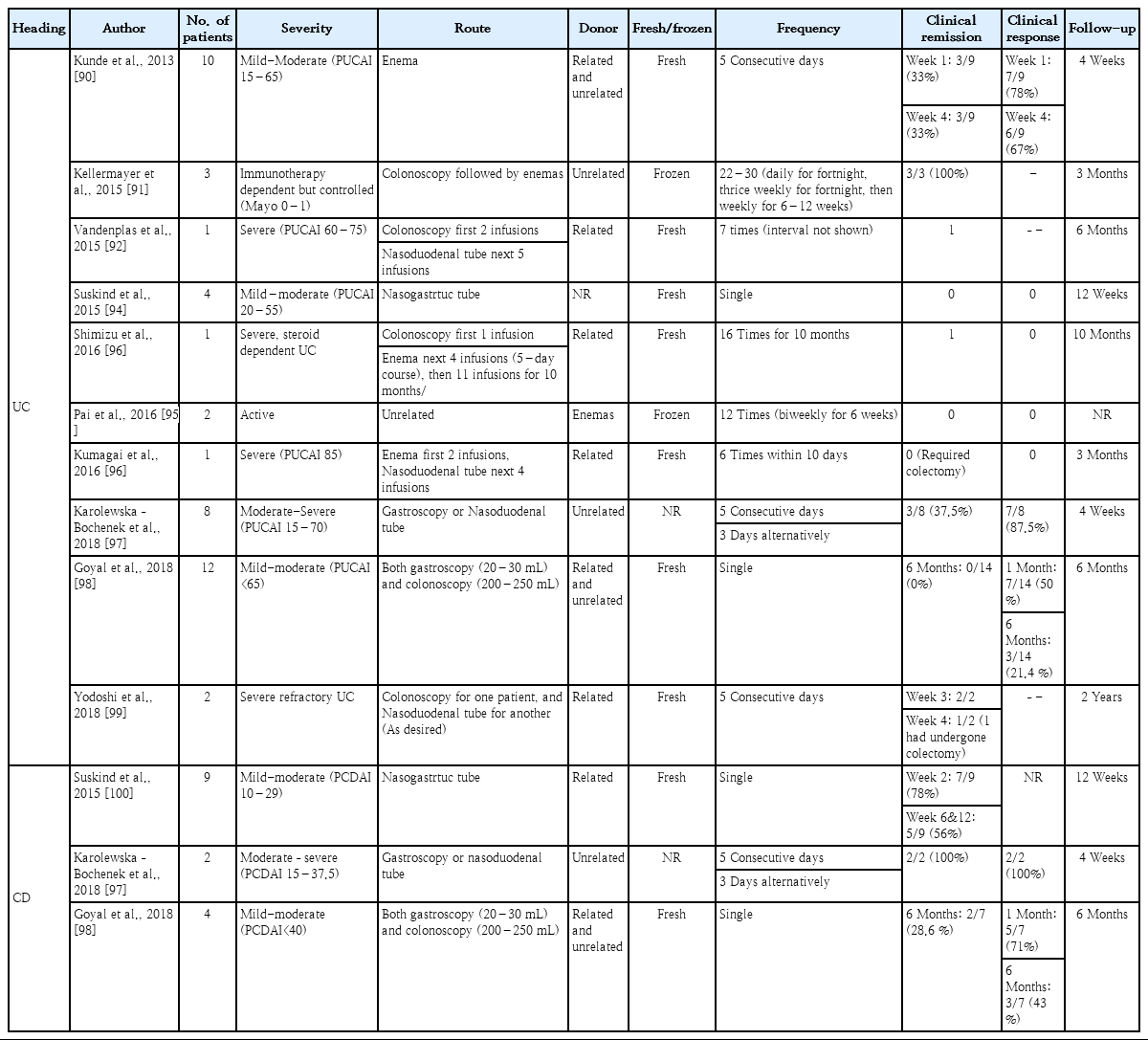
In pediatric patients, there are no published RCTs, but 6 cohort studies assessing 34 UC patients and 2 cohort studies assessing 13 CD patients showed a pooled estimated clinical remission rate of 23% (95% CI, 7%–51%) in UC and 54% (95% CI, 28%–78%) in CD patients [88]. The clinical trials and their results of FMT in pediatric IBD patients are summarized in Table 2 [90-100].
When there was a thorough investigation of donor fecal material, FMT has been considered a relatively safe treatment modality with a low rate of adverse events. Any reported side effects were mild and self-limiting [101]. Although serious adverse events (SAEs) such as flares were reported in several studies, a recent meta-analysis showed no significant difference between the FMT and the control groups with respect to SAEs. The pooled rate of SAEs was 7.1% in the FMT group versus 5.1% in the control group with no significant difference (risk ratio adverse events, 1.40; 95% CI, 0.55–3.58; P=0.49) [102].
FMT can be considered a relatively safe and effective treatment option for IBD that modulates the intestinal microbiota. However, several factors affecting FMT outcome and efficacy remain to be determined, including pretreatment, dosage and frequency, preparation of donor stools, and routes of administration. Further studies are needed to increase our understanding of and optimize FMT in IBD [12].
Conclusion
Numerous environmental factors can contribute to the increasing incidence of IBD. Of them, dietary and nutritional factors play an important role in the pathophysiology directly or through changes to the intestinal microbiota. In this “biologic era” in which a variety of new potent medications is being used, modifying causative environmental factors is still expected to play a more fundamental role in treating and preventing IBD. To increase our understanding of IBD, it is necessary to focus on the medical management and elucidate the environmental factors such as dietary treatment and FMT.
Notes
No potential conflict of interest relevant to this article was reported.