Neonatal seizures: stepping outside the comfort zone
Article information
Abstract
Seizures are the most common neurological disorders in newborns. Managing neonatal seizures is challenging, especially for neurologists who are not neonatal specialists. Acute brain injury during ischemic insult is a key component of seizure occurrence, while genetic and metabolic disorders play less prevalent but more severe roles. The diagnosis of neonatal seizure is ambiguous, as the subjective differentiation between seizure and nonepileptic events is difficult; therefore, electrographic recording is the gold standard for diagnosis. The detection of electrographic seizures by neonatologists is currently facilitated by amplitude-integrated electroencephalography availability in many neonatal intensive care units. Although it is less sensitive than conventional electroencephalography, it is better to record all risky neonates to filter the abnormal events as early as possible to enable the initiation of dedicated therapy at proper dose and time and facilitate the initial response to antiepileptic drugs. This, in turn, helps maintain the balance between unnecessary drug use and their neurotoxic effects. Moreover, the early treatment of electrographic seizures plays a vital role in the suppression of subsequent abnormal brain electricity (status epilepticus) and shortening the hospital stay. An explicit understanding of seizure etiology and pathophysiology should direct attention to the proper prescription of short- and long-term antiepileptic medications to solve the challenging issue of whether neonatal seizures progress to postneonatal epilepsy and long-term cognitive deficits. This review addresses recent updates in different aspects of neonatal seizures, particularly electrographic discharge, including their definition, etiology, classification, diagnosis, management, and neurodevelopmental outcomes.
Key message
∙ Use conventional and amplitude-integrated electroencephalography to confirm clinical seizures and screen high-risk newborns.
∙ Select an explicit clear elective event to be treated with less toxic and more effective antiepileptics.
Graphical abstract
Introduction
Seizure is the most common and critical neurological disorder in newborns [1]. Its incidence ranges from 1/1,000 to 5/1,000 live births in population-based studies [2-5] and 8.6/1000 live births in a neonatal intensive care unit (NICU)–based study [6]. Approximately 80% of neonatal seizures occur in the first week of life [7]. However, this subject remains a mystery due to dissimilarities and special characteristics of neonates versus older age groups and the fact that most seizures are subclinical without overt clinical correlates [8].
Neonatal seizure is a paroxysmal stereotypic repetitive event due to abnormal electrical activity of the brain caused mainly by ischemic or hemorrhagic insult [9]. The duration of this electrographic event remains under debate, as the American Clinical Neurophysiology Society defines neonatal seizures as an abnormal repetitive evolving (in frequency, voltage, and morphology) electrographic pattern lasting for at least 10 seconds [10], while brief rhythmic discharges lasting less than 10 seconds cause special concern as they have worse outcomes than electrographic events [11]. Therefore, the International League Against Epilepsy (ILAE) defines seizure as an abnormal evolving electrical signal with definite onset and offset; its duration is not necessary 10 seconds but should be long enough to detect changes in signal frequency, morphology, and resolution [12].
Despite the silent nature of electrographic seizures, they have gained special consideration in recent years, as in the selected high-risk hypoxic ischemic encephalopathy (HIE) group, 50%–80% of seizures are electrographic only and the extent of seizure burden may be greatly underestimated [13-15]. Moreover, they may adversely affect the brain and increase morbidity and mortality with increased risk of death, severe cerebral palsy (CP), microcephaly, and failure to thrive. Long-term neurological follow-up of these patients with electroclinical and electrographic-only seizures is poor, with significant morbidity and mortality rates [14].
Therefore, it seems reasonable to depend on electroencephalography (EEG) to accurately diagnose neonatal seizures and overcome the dilemma of clinical epileptic and nonepileptic events, and polygraphic video EEG can help to evaluate any manifestations in question, such as autonomic features or automatisms, and decrease the risk of overdiagnosing common nonseizure events as epileptic [16].
Diagnosis of neonatal seizures
Variability in the clinical presentation of neonatal seizures is the major factor of its misdiagnosis since it depends on clinical symptoms only, which may lead to overdiagnosis (excessive abnormal movement such as jitteriness, startle) or underdiagnosis (presence of electrical activity in areas away from the motor cortex) [17]. Therefore, the clinical suspicion of seizures should be verified by conventional/amplitude-integrated EEG (EEG/aEEG) recording whenever available. Of note, conventional EEG remains a cornerstone for diagnosis but cannot be used for long-term monitoring; thus, aEEG is a less sensitive but easily applicable and interpretable alternative in many centers [18].
A main obstacle is that EEG may miss some clinical events without recording due to the reduced montage of EEG in neonates, as only 9 electrodes are placed and focal seizures originating from subcortical cerebral areas such as the limbic and peri-limbic systems may be missed on EEG. However, studies have shown that the electrographic ictal pattern of epileptic seizures becomes apparent during prolonged EEG monitoring [14,19]. We must stress that clinical episodes that have no ictal EEG correlation are not seizures. The ambiguous “EEG-negative seizure” concept is eliminated [10] as seizures that do not originate from or migrate to the motor cortex do not result in obvious movements. Therefore, the placement of more EEG electrodes and continuous prolonged bedside electrographic monitoring are essential for interpreting EEG-negative seizures.
Another main obstacle is that EEG is not available in every NICU; therefore, it is better to follow the flowchart developed by the Brighton collaboration illustrating different degrees of diagnostic certainties (definite, probable, possible) [9] depending on the available tools. Therefore, a definite diagnosis is reached if an electrographic seizure is recorded by conventional EEG with or without clinical events, whereas probable certainty is reached if events are recorded on aEEG or focal clonic or focal tonic clinical events are witnessed. However, it is possible to ascertain whether other clinical events, such as automatisms, autonomic seizures, and seizures with behavioral arrest, are seen.
Notably, particular attention should be paid to the role of EEG as a screening tool for high-risk newborns and its application as early as possible to detect electrographic events even before their clinical occurrence. This idea has been studied by Wusthoff et al. [20], who found that seizures in HIE often lack distinct clinical signs and that preemptive use of conventional EEG for seizure screening increases treatment success compared to confirmatory EEG after clinically suspected seizures occur. Therefore, it is important for future research to redirect the goal of using conventional EEG as a tool to screen for seizures among high-risk neonates to facilitate an earlier and more accurate diagnosis and thereby more effective treatment avoiding unnecessary medication for seizure mimics.
Classification of neonatal seizures
Several years ago, Volpe [21] tried to classify seizures based on semiology only into tonic, clonic, myoclonic, and subtle types (staring, sudden awakening and alerting, eyeball deviation, eye blinking, nystagmus, chewing, and limb movements such as swimming, rowing, and pedaling), both focal and generalized. After the integration of EEG for the diagnosis, seizures are divided into electroclinical or electrographic only [22].
Electrographic seizure involves the presence of an electrographic seizure observed on EEG that is not associated with any evident clinical signs (synonyms: clinically silent or subclinical seizures). The term electrographic-only is preferred, as it depends on the observational methods used and the seizure may not be truly subclinical [12]. The diagnosis of clinical events in neonates is challenging for many reasons [23,24]. First, clear clinical events occur in a small number of neonates due to their immature motor pathways and underdeveloped central nervous system (CNS) connections [25] in term and preterm neonates; thus, the seizures may not spread to certain areas such as the motor cortex. Moreover, seizures that remain focal in the nonmotor cortex do not generate clear clinical events, which may hinder accurate segregation between seizures and nonepileptic movements [26].
Second, since newborns are preverbal, they are unable to communicate sensory phenomena associated with seizures, while a seizure in the temporal cortex may manifest as interrupted speech or in the occipital cortex may manifest as visual events that cannot be assessed. Third, neonates develop pseudo-paroxysmal events, such as jitteriness, rapid eye movements, automatisms, and sleeprelated myoclonus. Fourth, electroclinical “uncoupling” occurs after antiepileptic drug administration as clinical events stop but electrographic events continue, which may lead to premature discontinuation of antiepileptic drugs [27]. Finally, clinical observation of neonates in the NICU is often inhibited by isolette coverings, dark lighting, and other environmental features of modern neurocritical care. Therefore, the clinical signs of neonatal seizures are always subtle or even absent, and even the most attentive observer may miss them.
To our knowledge, the ILAE [12] recently proposed a diagnostic framework for the classification of neonatal seizures that consists of 4 zones: (1) clinical presentation: risk factor or clinically suspicious events; (2) diagnosis: EEG recording; (3) manifestation: with or without clinical events; (4) seizure types: motor: automatisms, clonic, epileptic spasms, myoclonic, sequential, and tonic; and nonmotor: autonomic and behavioral arrest; unclassified or electrographic only. The special consideration for this classification is that all neonatal seizures start with focal onset with special concern about unique sequential type, clinically only events are eliminated, and an impaired or preserved level of consciousness is unnecessary.
Etiology and clinical course
Etiological drives for neonatal seizures are related to acute brain insult, such as stroke, hemorrhage, infection, or electrolyte disturbance. Their onset usually occurs within one week after a dedicated event and are considered acute symptomatic/provoked seizures [28] or related to neonatal structural, metabolic, or genetic epileptic syndromes [29] that occur in the absence of a potentially causative clinical condition or beyond the interval of an acute event [30]. These represent approximately 15% of causes and are known as unprovoked seizures [31]. Coronavirus disease 2019 (COVID-19) infection remains uncommon in neonates, and little is known about its neurological complications. Neurological manifestations are mainly parainfectious and immune-mediated; Martin et al. [32] reported the first case of neonatal seizures due to COVID-19-induced encephalitis with good response to antiepileptic drugs and a normal short-term outcome. Although the clinical course is known to begin more with favorable outcomes than those of adults, one study showed an unusual presentation of COVID infection in full-term babies, including multisystem involvement and cerebral ischemic lesions [33]. In addition, 2 neonates exposed to prenatal infection developed ventriculomegaly, neurological dysfunction, and seizures [34]. A recent study emphasized that the COVID-19 pandemic has led to a widespread disruption in the delivery of developmental services to children with a history of neonatal seizures [35].
Of note, seizure onset and type are crucial to identifying the underlying etiology and hence tailoring early distinct therapeutic interventions aimed at reducing the detrimental effects of seizures on synaptic plasticity in the growing brain. HIE accounts for approximately two-thirds of acute symptomatic seizures occurring on the first day of life, whereas genetic disorders occur around the seventh day of life [36,37].
The clinical course of seizures beyond the neonatal period is challenging. There are 4 clinical scenarios (A, B, C, and D) according to brain injury etiology and severity [38]. The clinical courses of acute symptomatic seizures are completely within in the neonatal period (A), waking up in the postneonatal period within the first year of life [39,40] after the latent period of control (C), or continued without control, particularly in severe brain injury such as infectious encephalitis and severe HIE (D), whereas structural or genetic epilepsy is a remote cause of symptomatic seizures (B). To some extent, seizure onset in neonatal epilepsy overlaps with acute provoked events, as it may be superimposed onto acute brain injury [28].
Treatment
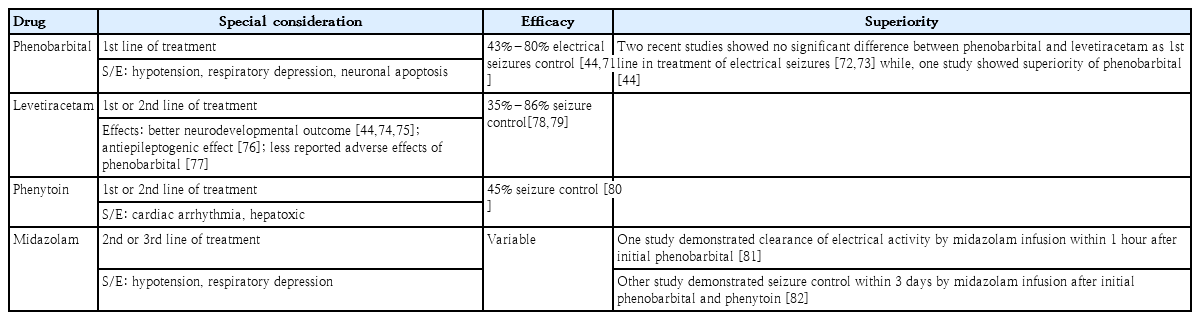
Treatment protocols for neonatal seizures have not significantly changed in recent decades, although they only are initial responders [41]. First-line therapy that acts through the gammaaminobutyric acid (GABA) receptor, that is, phenobarbital and benzodiazepines. Phenytoin or levetiracetam as second-line treatment has not yielded superior results [42]. Levetiracetam is attractive for its neuroprotective effect [43], although a recent study suggested poor seizure termination efficacy compared to phenobarbital [44].
Moreover, many centers use midazolam infusion for persistent events [42]. Lidocaine is also an alternative in refractory cases if phenytoin has not been previously utilized [45]. Alternative drugs, such as topiramate, have more limited use because of the unavailability of an intravenous formulation despite its neuroprotective effect [46]. It acts by inhibiting α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and kainate receptors and potentiating GABA signaling, resulting in dose-dependent brain injury reduction and neurobehavioral improvement. In addition, when combined with hypothermia, topiramate use was correlated with a lower seizure burden during therapeutic hypothermia, less need for drug titration, lower mortality rates in newborns with HIE, and a lower prevalence of epilepsy [47]. Table 1 summarizes the advantages and disadvantages of commonly used antiepileptic drugs for the treatment of neonatal seizures.
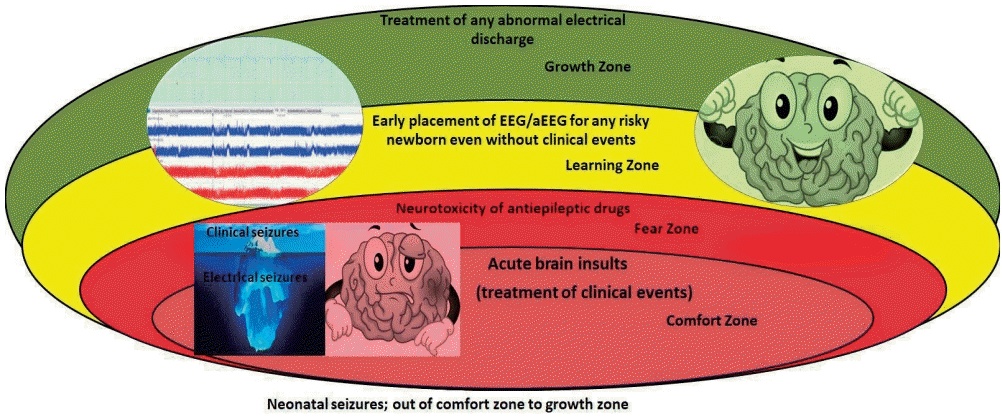
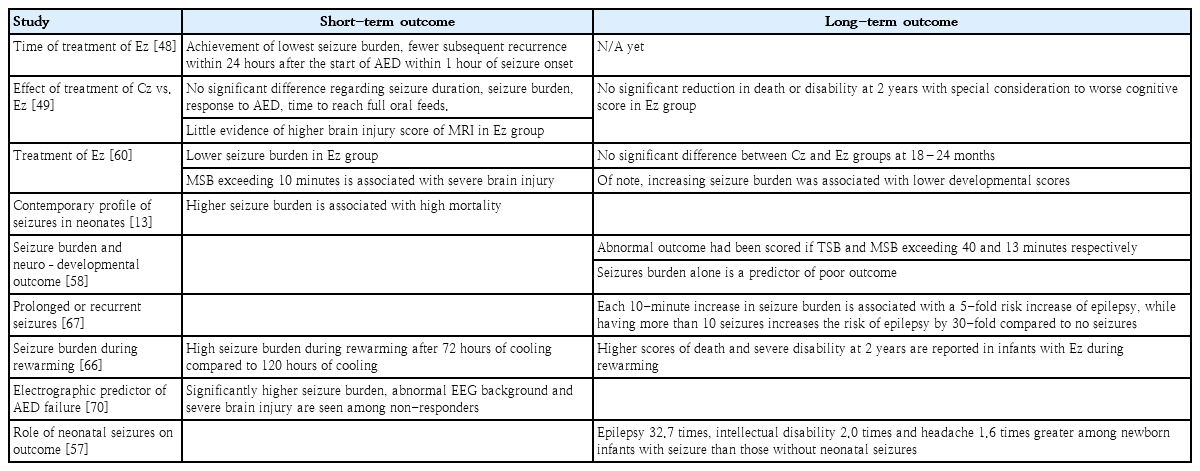
Clinicians and researchers have begun to widen the comfort zone for the treatment of neonatal seizures, weighing the balance between benefits and harms by reducing the seizure burden and drug neurotoxicity. Therefore, recent studies paid great attention to the treatment of electrographic seizures even without clinical events as it is the common presentation in neonates [26]. The treatment of electrographic seizures is ideally time-critical, as infants treated within 1 hour of seizure onset had the lowest seizure burden and fewer seizures over the subsequent 24 hours [48]. However, a recent study reported no difference in mortality or long-term morbidity in neonates treated after clinical or electrical events with some concern with worse cognitive function in those with electrographic seizures [49]. Briefly, the threshold for escalation of therapy to decrease seizure burden remains an area of active research.
The duration of drug continuation after seizure resolution remains variable [50] but accumulating evidence supports their earlier discontinuation before hospital discharge, although exact timing has not been reported [42] if acute symptomatology is confirmed, as it will not be associated with the risk of childhood epilepsy or worse neurodevelopmental outcomes [51]. Interestingly, Vegda et al. [52] designed an algorithm for medication discontinuation based on the risk of recurrence and availability of EEG/aEEG; therefore, seizures that are controlled with one drug and resolved within 2–3 days, it is reasonable to withdraw and stop the medication prior to discharge. If the seizures exceed 7 days, require more than 2 drugs for control, or are confirmed as epilepsy, it is reasonable to maintain the baby on 1 or 2 drugs at discharge and refer them for following by a neurologist to decide when to wean the medication.
Outcome
Acute symptomatic seizures have been identified as a major risk factor for neurological and cognitive impairment as well as the development of epilepsy in children in developing countries [53]. The main prognostic factors associated with poor outcomes are prematurity, low birth weight, low Apgar score, severe HIE, high-grade intraventricular hemorrhage, abnormal EEG background in the form of burst suppression or long-term low voltage, seizure onset on the first day or that persisted after the third day of life, status epilepticus, CNS infection, and brain damage [9,54]. The most common neurological sequelae of neonatal seizures include developmental delay (30%–50%) [9], epilepsy (20%–35%) [9], and CP (15%–30%) [9]. Mortality correlates with the etiology of seizures (7%–25% in neonates with seizures) [9], the highest mortality rate is related to HIE, hemorrhage, and stroke [13].
Seizure etiology, burden, and response to medications are the key factors of outcomes (Table 2) [55]. The underlying causes of seizures is considered the critical determinant of neurological abnormalities later in life, as the relative risk for adverse outcomes in neonates with perinatal asphyxia was 8.41 while that for neonates with stroke and seizures was 4.95 compared to that of those with stroke but no seizures [56]. Of note, Oh [57] estimated the impact of neonatal seizures on subsequent neurological disorders, such as epilepsy, intellectual disability, psychiatric and behavioral disorders, and headache, independent of the putative underlying etiology. They found that the risk of developing epilepsy, intellectual disability, and headache among newborn infants with seizures was 32.7, 2.0, and 1.6 times that among those without neonatal seizures, respectively. Surprisingly, the risk of subsequent epilepsy was considerably higher in infants who were prescribed antiepileptic drugs.
Moreover, there is a positive relationship between seizure burden and brain injury severity [57]; a maximal seizure burden exceeding 13 min/hr is associated with neurological morbidities in HIE infants [58]. Further studies found that electrographic seizure burden exceeding 10 minutes is significantly associated with severe brain injuries [55,59,60]. Surprisingly, seizure burden is eliminated with an early start of dedicated therapy and antiepileptic drugs, resulting in a reduction in the development of status epilepticus [61,62] and better cognitive outcomes [60].
Of note, many studies reported a high incidence of seizures during cooling and rewarming periods in cooled HIE newborns [24,63-65]. Interestingly, Chalak et al. [66] found a higher seizure burden during rewarming and worse long-term outcomes at 2 years of age and highlighted the importance of continuous EEG monitoring during the rewarming period. In addition, a longer seizure duration is associated with a higher 1-year risk of epilepsy in children with perinatal asphyxia, and each 10-min increase in seizure burden is associated with a 5-fold increase in the risk of epilepsy, while having more than 10 seizures increases the risk of epilepsy by 30-fold compared to no seizures [67].
Moreover, worse outcomes and higher mortality rates were scored in neonates with electrographic-only seizures versus those with clinical or electroclinical seizures (P<0.002), and the mortality rate was doubled in neonates who did not respond initially to the loading dose of antiepileptic drugs [13]. The number of electrographic seizures (none, 1–75, >75) correlates with subsequent mortality and morbidity in a cohort of at-risk newborns and newborns with HIE [68]. Background EEG and electrographiconly seizures were independent predictors of a lack of response to phenobarbital in one study [69]; moderately and severely abnormal EEG findings were associated with poor response in an additional study, which also identified higher mean seizure score and higher degrees of brain magnetic resonance imaging injury (white matter, cortex, and watershed regions) were associated with a poor response to phenobarbital [70].
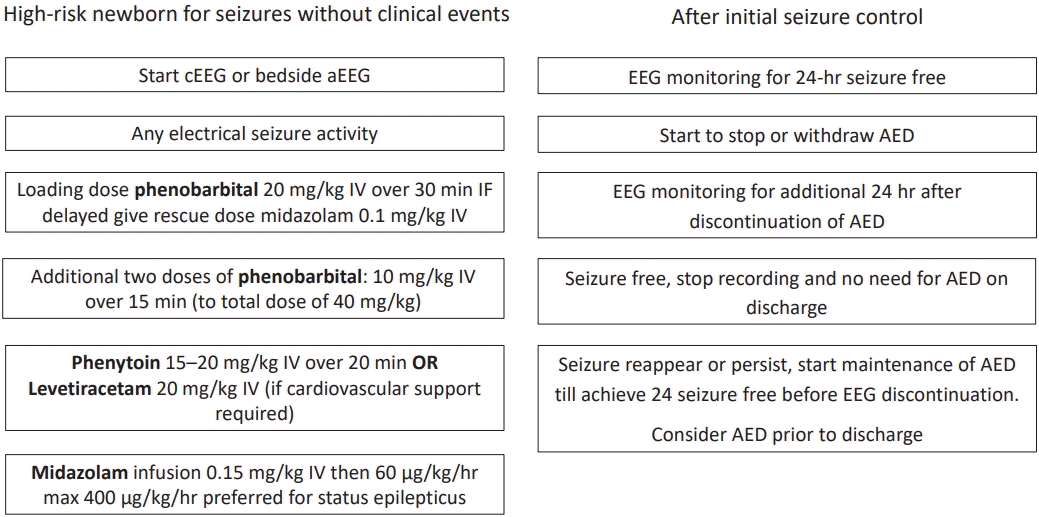
Briefly, the early declaration of this silent enemy is vital through the early use of EEG in any suspected or critically ill newborn with the aim of detecting any abnormal discharge and initiating antiepileptic drugs in curable doses that dampen the current and subsequent sparks. Moreover, it is reasonable to pause these drugs as soon as possible after seizure control is achieved due to their neurotoxic effects, with selective subsequent continuation of medication in non-responder babies. The flow chart in Fig. 1 illustrates the initiation and termination of antiepileptic drugs for electrographic seizures.
Conclusion
The threshold of neonatal seizure decreases; therefore, it is reasonable to consider EEG application as a confirmatory tool for clinical events as well as a screening tool for high-risk newborns. The approach to healthy brains after detrimental insult involves building a standardized framework allocating the classification of neonatal seizures based on their EEG signature, pathophysiologic-clinical-semiology features, and thus, selection of nominated antiepileptic drugs for a dedicated duration to overcome their adverse effects.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: MRB; Data curation: MH; Formal analysis: MRB; Visualization: MH; Writing original draft: MH; Writing review & editing: MH, MRB