Epidemiology and surveillance implications of community-acquired pneumonia in children
Article information
Abstract
Community-acquired pneumonia (CAP) is the single largest infectious cause of hospitalization and death in children worldwide. With improved immunizations, the incidence of bacterial pneumonia and the number of colonized bacteria have decreased. However, respiratory viruses are still an important cause of CAP, especially as new infectious agents such severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerge. The SARS-CoV-2 virus emerged in 2019 and caused the current coronavirus disease 2019 pandemic. Therefore, it is necessary to elucidate the epidemiology and causative pathogens of CAP. Recently, the Pneumonia and Respiratory Disease Study Group, affiliated with the Korean Academy of Pediatric Allergy and Respiratory Disease, investigated the causative pathogens of respiratory infections in children hospitalized with CAP, the serotype of Streptococcus pneumoniae, and the prevalence of Mycoplasma pneumoniae with gene mutations. Antibiotic resistance and serotype test results can determine the use of empirical antibiotics. Moreover, it is possible to help develop future vaccines by comparing bacterial culture results with vaccine serotype and identifying the changes and prevalence of each serotype. Therefore, we will perform continuous national surveillance and monitor the epidemiology of respiratory pathogens in Korea and worldwide. The surveillance of these respiratory infections can play a role in monitoring the emergence of new infectious diseases such as SARS-CoV-2.
Key message
The identification of the causative pathogens of community-acquired pneumonia and appropriate treatment and prevention can reduce mortality and the socioeconomic burden by reducing the medical expenses. The world has been in the coronavirus disease 2019 pandemic since 2020, and there is always a risk of continuous emergence and epidemic of new respiratory infectious diseases. Therefore, it is important to sustain a monitoring system for respiratory infectious diseases including pneumonia.
Graphical abstract. MRMP, macrolide-resistant M. pneumoniae; MSMP, macrolide-sensitive M. pneumoniae; KoCCAPS, Korean Children Community Acquired Pneumonia Study Group.
Introduction
Acute respiratory tract infectious diseases have a wide range of clinical features, ranging from mild diseases such as upper respiratory infections to severe diseases such as pneumonia and acute respiratory distress syndrome. Acute respiratory tract infections are characterized by easier and faster transmission than those of other diseases. As the aviation industry develops, new respiratory infections that occur abroad are more likely to infiltrate Korea. In addition, recent new infectious diseases are mainly respiratory infections with pneumonia as a major feature [1]. By strengthening the surveillance of respiratory infections, it is essential to identify the prevalence and severity of respiratory infectious diseases and recognize the early stages of new and mutated respiratory infections. In particular, among acute respiratory tract infections, monitoring for pneumonia identifies the causative pathogens and reveals the characteristics of the pathogens and antibiotic resistance through molecular biological examinations. This can also serve as basic data for the national policy for managing infectious respiratory diseases. The World Health Organization recommended the monitoring of severe acute respiratory infections for the early blocking and preemptive response to new infectious diseases after the 2009 influenza epidemic [2,3]. The United States has operated a monitoring system for severe acute respiratory infections since 2009, China and Europe since 2010, and New Zealand since 2012 [4-6]. Accordingly, Korea has continued to monitor infectious diseases. The Korea Centers for Disease Control and Prevention Agency established and has operated a nationwide respiratory infectious disease monitoring network since 2015 that monitors respiratory infectious diseases and identifies the causative pathogen. This monitoring network identified the trend of respiratory infections in Korea [7,8].
Epidemiology
Pneumonia is the single largest infectious cause of death in children worldwide. Pneumonia killed 740,180 children younger than 5 years in 2019, accounting for 14% of all deaths of children under 5 years old and 22% of all deaths in children aged 1–5 years worldwide [9]. The mortality rate due to pneumonia in Korea is 1.8 per 100,000 people under 1 year of age and 0.1 per 100,000 people aged 1–4 years in 2020 (Fig. 1A) [10]. The epidemiology of child pneumonia varies widely among different regions of the world related to the prevalence of risk factors and causative pathogens [11]. Considering the prevalence of pneumonia by country, community-acquired pneumonia (CAP) accounts for 2 million outpatient visits annually in the United States (US) [12] and 2.5 million cases in Europe [13]. In Korea, the incidence of pneumonia for children under 5 years of age was 0.20–0.249 per child-year [11]. According to the Health Insurance Corporation Review and Assessment Service, about 1.34 million patients of all ages were treated for “pneumonia,” of whom half (47.2%) were children and adolescents [14].
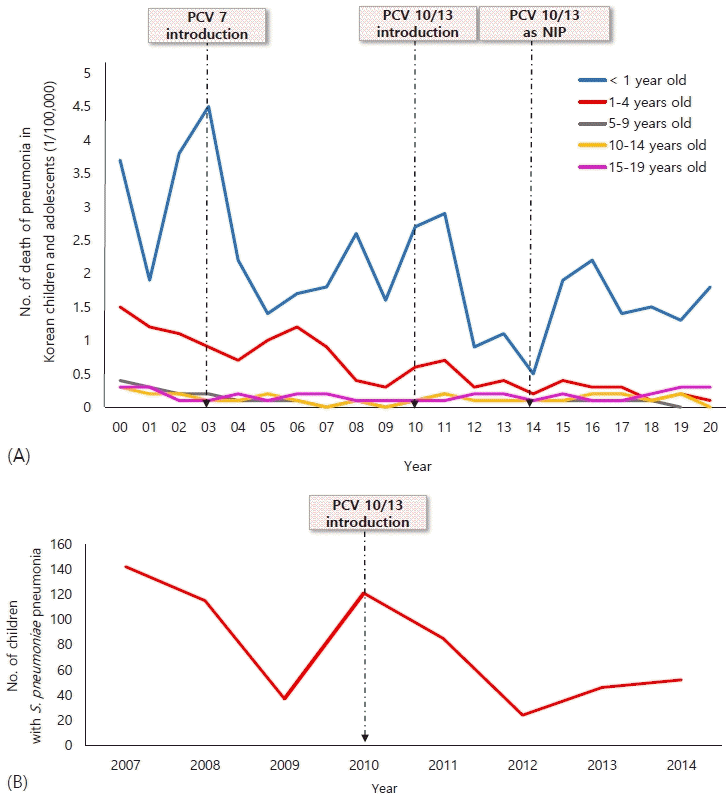
(A) Number of deaths of pneumonia in Korean children and adolescents reported by the Korean Statistical Information Service.10) (B) Number of children with Streptococcus pneumoniae pneumonia, 2007–2014.18,19) PCV, pneumococcal conjugate vaccine; NIP, National Immunization Program.
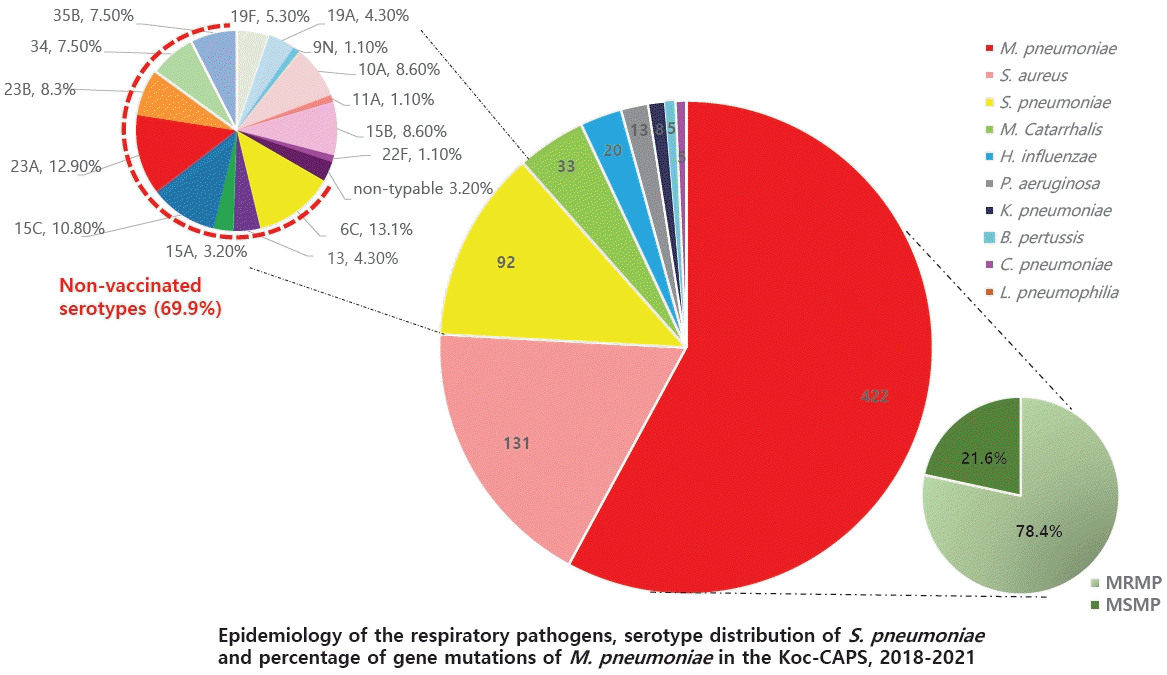
Globally, the incidence of child pneumonia decreased by 30% and that of mortality decreased by 51% during the Millennium Development Goal period [15]. This fact suggests that efforts are being made to prevent, recognize, and treat pneumonia. Improved healthcare access, vaccination programs, living conditions, and nutrition are key to further reducing CAP mortality rates [16]. In particular, the introduction of routine childhood vaccination against both Streptococcus pneumoniae and Haemophilus influenzae type b has dramatically reduced the diseases caused by these pathogens. Several studies of pneumonia in different age groups soon after introduction of the pneumococcal conjugate vaccine 7 (PCV-7) in Canada, Italy, Australia, Poland, and the US showed a decreased incidence of pneumonia hospitalizations (15%–65%) [17]. In 2003, the PCV-7 was first introduced in Korea; in June 2010, a 10-valent (PCV-10) and a 13-valent (PCV-13) were introduced. In May 2014, PCV-10 and PCV-13 were incorporated into the National Immunization Program in Korea. The frequency and mortality rate of pneumonia, including Streptococcus pneumoniae pneumonia, have rapidly decreased since 2014 (Fig. 1B) [18,19]. The PCV also reduces nasopharyngeal carriage of vaccine-type pneumococci, which also reduces the risk of one of the causative pathways of pneumonia [20]. In addition, the national pneumococcal vaccination program caused a serotype replacement phenomenon. Infection rates with the vaccine serotype decreased and the prevalence of the disease caused by serotypes that were not included in the vaccine increased. A study was performed of adults to investigate the serotype distribution of S. pneumoniae in Asian countries. The study showed the persistent prevalence of 19F and 19A with a noteworthy increase in certain non-PCV-13 serotypes in Asian countries [21]. A recent multicenter study analyzed the characteristics of 93 cases of S. pneumoniae through the child CAP monitoring project in 2018–2021. A total of 69.9% were non-vaccinated serotypes, and the 19A (4.3%) and 19F (5.3%) serotypes were included in the PCV10 and PCV-13 vaccines (Fig. 2) [22].
The introduction of routine childhood vaccination against Haemophilus influenzae type b has dramatically reduced the diseases caused by these pathogens. In a review of four randomized controlled trials and two case-control studies of H. influenzae type b conjugate vaccination in high-burden communities, vaccination was associated with an 18% decrease in radiologic pneumonia [23], while the rates of meningitis and laryngitis also decreased [24].
Detection of CAP pathogens
The diagnosis of pneumonia is generally possible through a thorough medical history, a physical examination, and chest radiography. It is also important to identify the causative pathogens for the proper treatment of pneumonia, which is also necessary to prevent antibiotic abuse. Pneumonia in a child is the condition for which the microbiological diagnosis is most difficult to determine. An estimated one-third of cases of pneumonia can be attributed to a specific etiology using culture, antigen detection, and serologic techniques [25]. A review of European pediatric studies found that, depending on the extent of laboratory testing performed, the microbial cause of pneumonia could be identified in 20%–60% of cases [13].
Bacteria as CAP pathogens
Blood cultures identify pathogens in only 2%–7% of children with CAP [26]. The estimated microbial yield of blood cultures in pediatric pneumonia is 2% when the blood culture volume is 1 mL or less and increases to 6% or more for volumes of 3 mL or more [27]. The isolation of pathologic organisms occurs significantly less frequently in patients exposed to antibiotics prior to the specimen collection [28]. Therefore, in the outpatient setting, blood cultures are not routinely recommended. Sputum cultures have low diagnostic yield in children due to the inability of most young children with pneumonia to produce adequate sputum samples. Therefore, sputum culturing should be attempted in older children and adolescents with more severe disease, including inpatients and those in whom outpatient therapy has failed [29].
Up to two-thirds of children younger than 5 years of age are colonized in the upper respiratory tract with common bacterial pathogens known to cause pneumonia [30], and polymerase chain reaction (PCR) analysis of samples from the upper respiratory tract is not a reliable method for ascertaining the bacterial etiology of pneumonia. Nasopharyngeal colonization with S. pneumonia or H. influenzae is common in young children and generally asymptomatic, and only a fraction of those colonized develop the disease [31]. The colonized bacteria in the nasopharynx can spread to others through droplets, causing infection. In addition, the serotype of pneumococcus separated from the pharynx is generally similar to that of otitis media infection, spreading from the nasopharynx to the middle ear through the Eustachian tube [30,32]. Therefore, S. pneumoniae or H. influenzae identified in the upper respiratory tract may or may not be the causative pathogen of pneumonia diagnosed at the time, so it is important to determine its clinical relevance.
In schoolchildren and adolescents, other important bacterial pathogens include M. pneumoniae and Chlamydophila pneumoniae [33]. To determine the possible role of M. pneumoniae and C. pneumoniae in the etiology of pneumonia, serological tests and PCR tests are commonly used. When these two methods are used together, the sensitivity increases to 95% [34]. However, the two primary methods for detecting these pathogens in the clinical setting could produce discordant results according to disease stage. Positive PCR tests often occur in the presence of negative serologic tests in the early infection stages [35]. Sometimes, when atypical pneumonia pathogens are identified by PCR in the upper respiratory tract, their causal role in pneumonia can be difficult to determine, especially in the lack of an epidemic. One study examined the prevalence of C. pneumoniae in healthy children without evidence of respiratory infections and in ill children. Although positive results were obtained most often in sick children, positive results were also obtained in healthy children (38%–51% vs. 11%–13%) [36]. Thus, a positive PCR result for C. pneumoniae in the upper respiratory tract does not necessarily imply that it is the etiologic agent of pneumonia.
Respiratory viruses as CAP pathogens
Respiratory viruses are the most common cause of CAP in children younger than 5 years of age, the incidence of which decreases as age increases. Viruses alone account for up to 50% of cases in young children [33]. The respiratory syncytial virus (RSV) is the most common viral cause of CAP, especially in hospitalized young children. Global data from 2015 showed that RSV accounted for approximately 36,000 pneumonia deaths in children under 5 years of age, approximately 20% of pneumonia cases [37]. Pneumonia with human metapneumovirus (HMPV) has the highest prevalence (44%) in infants younger than 12 months [38]. The prevalence of adenovirus (ADV) pneumonia is fairly low, but it is important to recognize because it causes severe and fatal necrotizing pneumonia [39]. ADV has 51 serotypes, of which types 3, 7, and 21 are known to cause pneumonia in children. There was also a severe ADV pneumonia epidemic of types 3 and 7 in Korea [40,41].
PCR tests for viruses from upper respiratory samples have been used universally because of their superior sensitivity, rapid turnaround time, and ability to identify viruses that are difficult to culture [42]. Despite technological advances, establishing the cause of pneumonia remains challenging. Specimens from the lower respiratory tract can be difficult to obtain, and distinguishing colonization from infection can be difficult. The detection of a virus in the nasopharynx could represent a coincidental upper respiratory infection or a pneumonia pathogen [43]. It can also be detected in healthy asymptomatic children; therefore, it may not be the causative pathogen. In addition, even if an individual suffered from respiratory infections 1–2 weeks prior, the virus can be detected by shedding for a long time. Therefore, to determine the detected virus as the causative pathogen, it is important to consider it with any clinical findings [43]. It is also important to consider the causative pathogen depending on the type of respiratory virus detected. Some viruses detected in the upper respiratory tract may cause lower respiratory tract disease (e.g., RSV, influenza virus [IFV], and HMPV) but, other viruses (e.g., human rhinovirus [HRV], coronavirus [CoV]) must be interpreted with caution [44]. Similarly, the PERCH study showed that RSV, parainfluenza virus (PIV) type 1, HMPV, when found in nasopharyngeal secretions were likely a cause [20]. Other casecontrol studies showed that RSV or IFV are strongly associated with pneumonia [37]. With the advent of PCR techniques, HRV has been detected increasingly in childhood pneumonia cases [45], but its role in pneumonia is still questioned because of the frequent detection of HRV in asymptomatic individuals (mean prevalence, 15%), strikingly more frequently than other respiratory viruses (prevalence, 1%–5%) [46]. On the other hand, we should consider the possibility of unknown new pathogens that we cannot yet detect.
Worldwide studies of causative pathogens of CAP
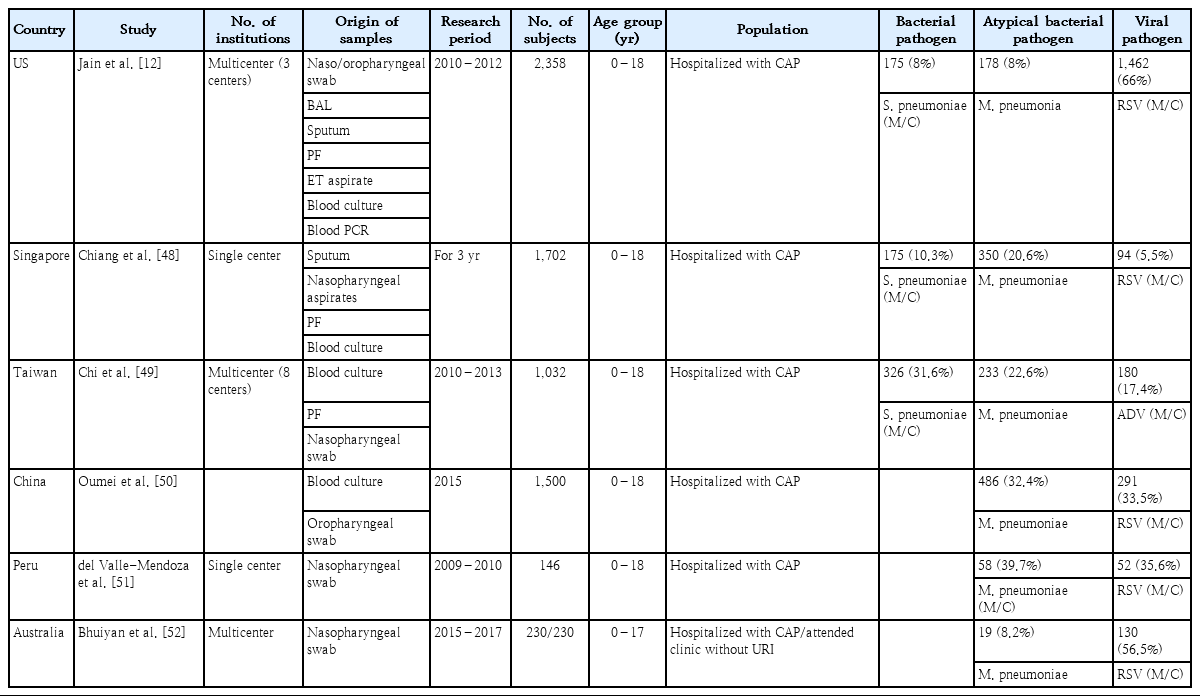
Most studies of the causative agents of CAP in children are limited by difficulty obtaining adequate specimens. Several studies have investigated the causative agents of CAP (Table 1). In a 3-year US-based study (2010–2012), the causative pathogen was identified in 81% of children hospitalized with CAP: viruses in 66% versus bacteria in 8%. The most commonly detected viral pathogens were RSV (28%), HRV (27%), HMPV (13%), and ADV (11%), while the most common bacterial pathogens were M. pneumoniae (8%), S. pneumoniae (4%), and Staphylococcus aureus (1%), and Streptococcus pyogenes (<1%).12) In this study, PCR tests targeting S. pneumoniae (lyt-A) and S. pyogenes (spy) genes were performed on whole blood and pleural fluid [47].
In a study published in Singapore in 2007, causative pathogens were identified in 38.4% of children hospitalized with CAP, including M. pneumoniae in 20.6%, other bacteria in 10.3% (S. pneumonia in 64.6%, nontypeable H. influenzae in 21.7%), and viruses in 5.5% [48]. In a study of Taiwan (2010–2013), the pathogen detection rate was 68.3%, and S. pneumoniae (31.6%) was the most common pathogen, followed by M. pneumoniae (22.6%), ADV (5.9%), and mixed viral-bacterial infection in 10.2% [49]. In a Chinese study of 1,500 children hospitalized with CAP in 2015, 46.1% tested positive for at least one pathogen; M. pneumoniae (32.4%) was detected most frequently, followed by RSV (11.5%) and ADV (5%) [50]. In a Peruvian study (2009–2010), M. pneumoniaewas the most common etiologic agent of CAP, followed C. pneumoniae, and the most frequent respiratory viruses detected were RSV, IFV, and PIV [51]. In an Australian case-control study of pneumonia (2015–2017), S. pneumoniae, H. influenzae, and Moraxella catarrhalis were detected at similar frequencies in children with versus without pneumonia. However, high vaccine coverage has almost eliminated pathogenic vaccine-type strains of S. pneumoniae and H. influenzae from this population [52].
Korean studies of CAP causative pathogens
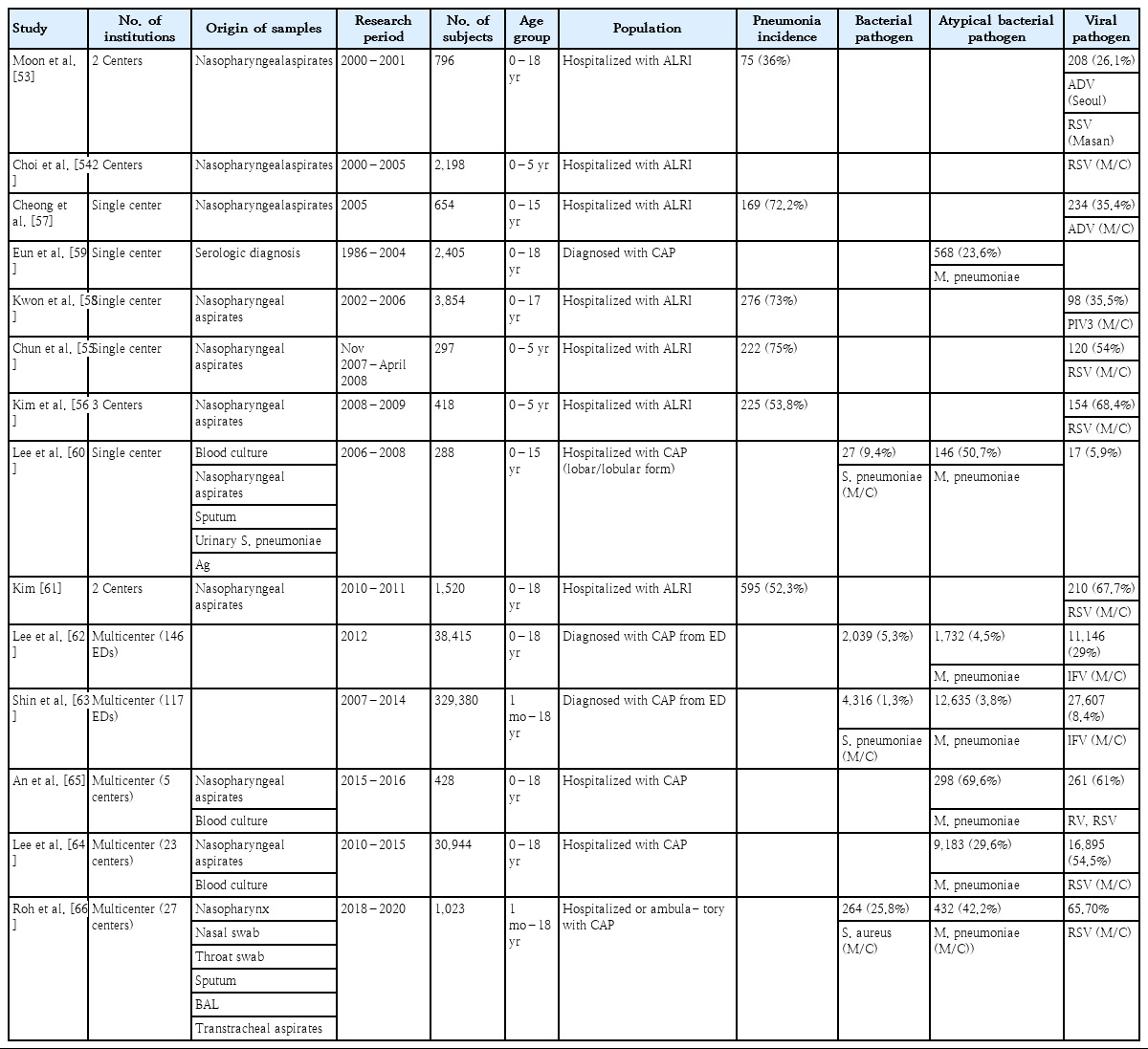
Several studies have been conducted on CAP pathogens in Korea (Table 2). One study examined 796 hospitalized children with acute respiratory infection at two hospitals and found that the most frequent viral pathogens causing pneumonia were ADV in Seoul and RSV in Masan [53]. In three studies of children under 5 years of age hospitalized for acute lower respiratory tract infection, RSV was the most common cause of pneumonia [54-56]. Among pediatric patients hospitalized for acute lower respiratory tract infection, the prevalence of pneumonia was approximately 70%: ADV was detected most in 2005 in Cheonan and PIV3 in 2002–2006 in Seoul [57,58]. An analysis of 2,405 patients with pneumonia at a single center for 18 years showed a 23.6% incidence of M. pneumoniae infection [59]. In a study of the causative agent of lobar and lobular pneumonia in a single institution in 2006–2008, M. pneumoniae was the most frequent (50.7%), followed by other bacteria (9.4%) and viruses (5.9%). Among the bacterial pathogens, S. pneumoniae was prevalent (88.9%) [60].
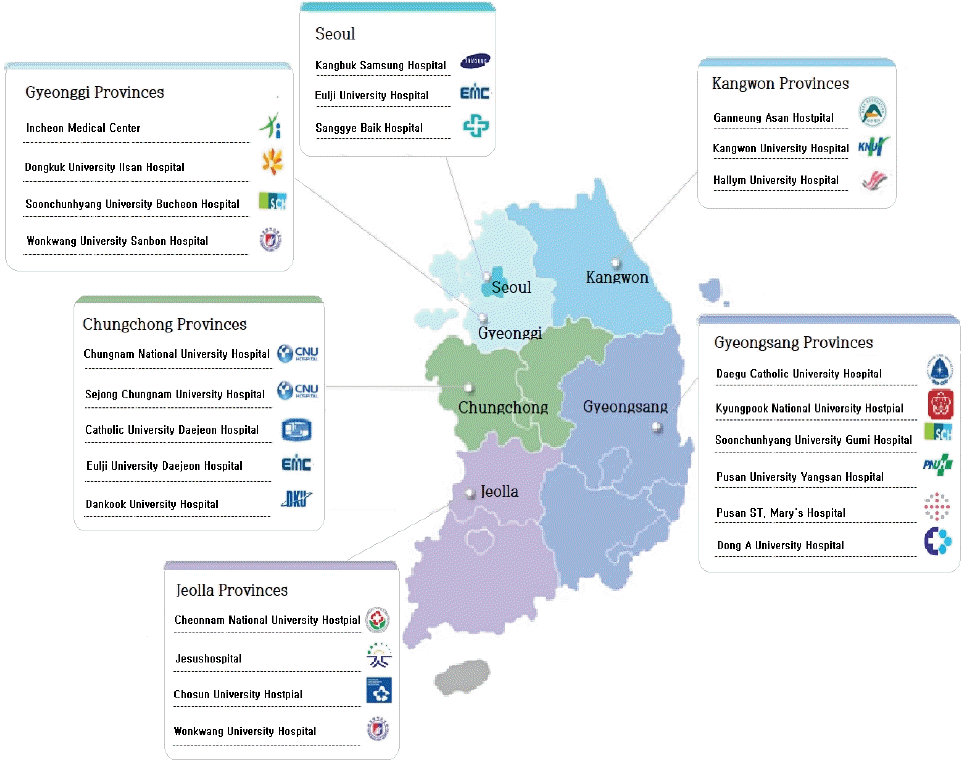
Among 1,520 children hospitalized with acute lower respiratory tract infection in Busan and Gyeongsangnam-do, pneumonia was the most common (52.3%), of which RSV caused 67.7% [61]. Among children who visited 146 Emergency Departments (EDs) due to CAP in 2012, viral pneumonia was prevalent (29%), followed by bacterial pneumonia (5.3%) and M. pneumoniae (4.5%) [62]. Another study of children with pneumonia visiting EDs in 2007–2014 showed viral pneumonia comprised 8.4%, M. pneumoniae pneumonia comprised 3.8%, and bacterial pneumonia comprised 1.3%: IFV was the most prevalent (41.8% of viral pneumonia), followed by RSV (17.3% of viral pneumonia) [63]. Because of the influenza H1N1 pandemic in 2009 and 2010, in two multicenter studies of hospitalized children with CAP, RSV and M. pneumoniae were the most commonly identified pathogens of CAP [64,65]. Various causative pathogens of pneumonia have been reported depending on region, age group, time period, test method, or sample type collected. We recently reported a nationwide study of the causative pathogen of CAP in Korean children and adolescents [66]. The study was performed at a cooperative hospital monitoring network composed of secondary and tertiary hospitals in 6 metropolitan areas (Seoul, Gyeonggi Province, Chungcheong Province, Gangwon Province, Jeolla Province, Gyeongsang Province).
The Korean Childhood Community-Acquired Pneumonia Study Group (KoC-CAPS) was established and CAP pathogens of CAP in hospitalized children from August 2018 to June 2020 (Fig. 3). Of the 1,023 children, the rate of pathogen detection was 70.5%; viruses were highest (65.7%), followed by atypical pneumonia pathogens (42.2%). Among viruses, HRV was the highest (29.8%), followed by RSV (20.3%), ADV (11.8%). Since February 2020, when the COVID-19 epidemic began, the rate of respiratory infections has decreased sharply. In particular, since February 2020, the number of patients with pneumonia has decreased sharply because of restrictions on group activities as well as high-intensity social distancing, mask-wearing, and handwashing. Additionally, the indefinite postponement of openings at kindergartens and schools, the prevalence of homeschooling, and a decrease in air pollutants have also been important factors in the decrease in respiratory tract infections [67]. Fewer HRV, ADV, and bocavirus cases were detected, while RSV, IFV, PIV, and HMPV were not detected at all after the COVID-19 outbreak (Fig. 4). ADV and HRV activity continued during 2020 and might be returning to prepandemic circulation patterns [68-70]. Factors contributing to this distinct circulation are unclear but might include the relative importance of different transmission mechanisms, such as aerosols, droplets, or contact, the role of asymptomatic transmission, and prolonged survival of these non-enveloped viruses on surfaces. This may make these viruses less susceptible to nonpharmaceutical interventions such as mask-wearing and surface cleaning [71,72]. In 2021, as the number of visits to daycare centers and schools increased, the incidence of PIV infection and PIV pneumonia also increased. Since December 2021, rates of RSV bronchiolitis and pneumonia have also increased [7,8].
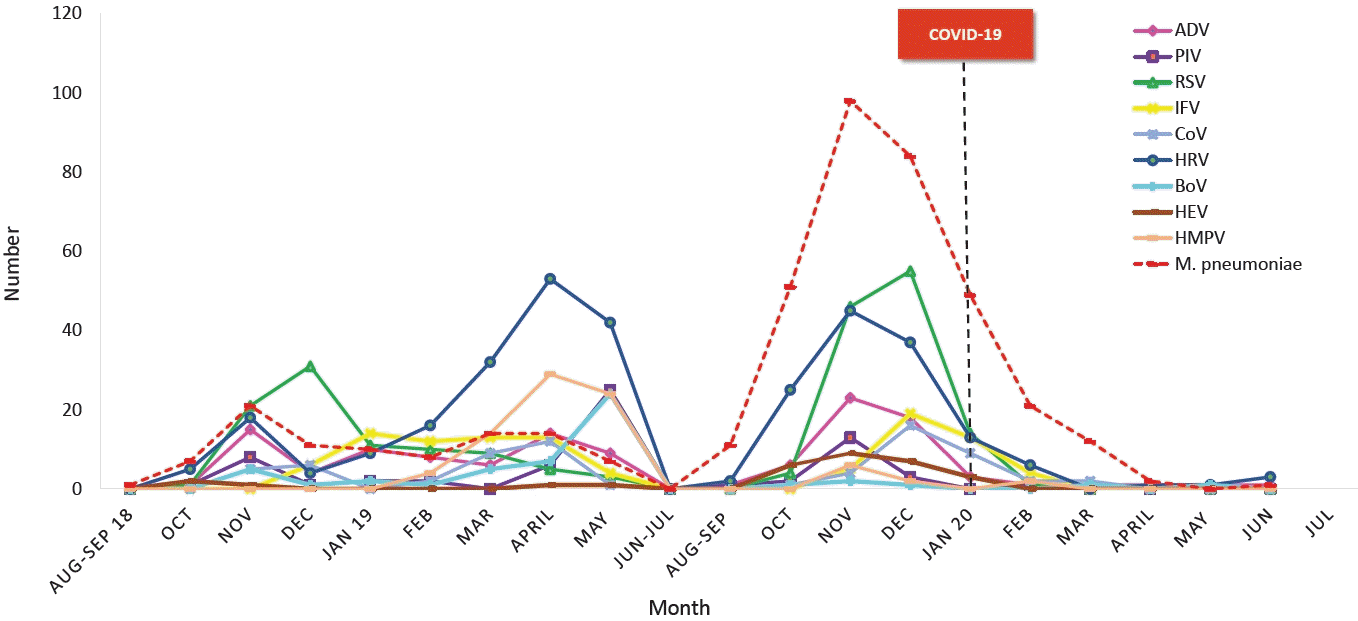
The annual patterns of the respiratory viral and atypical pneumonia pathogens in children with community-acquired pneumonia reported from the Korean Children Community Acquired Pneumonia Study Group.[66] COVID-19, coronavirus disease 2019; ADV, adenovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; IFV, influenza virus; CoV, coronavirus; HRV, human rhinovirus; BoV, bocavirus; HEV, human enterovirus; HMPV, human metapneumovirus; M. pneumoniae , Mycoplasma pneumoniae.
M. pneumoniae was prevalent among atypical pathogens (Fig. 5A) [66], and 78.4% of M. pneumoniae cases were caused by macrolide-resistant mutated strains; A2063G mutation in domain V of 23S rRNA (Fig. 5B) [73]. Previous studies have reported that the macrolide-resistant M. pneumoniae (MRMP) rate has been rising continuously and rapidly in Korea. The MRMP rate in 2011 was 51.6%–62.9% [74,75], but 60%–87% of children reportedly had the A2063G mutation during 2018–2020 in Korea [76-78], which is in agreement with the findings of the present study. M. pneumoniae has a high prevalence in Asia, including in Korea and Japan. Because Asia has a higher population density than Europe or the US, it spreads easily within families and schools. In addition, because the MRMP ratio is high, especially in eastern Asian countries such as China, Korea, and Japan, macrolide treatment does not kill bacteria, which may lead to more human-to-human transmission. The reason for the high MRMP rate seems to be the large number of resistant bacteria owing to the overuse of macrolides in Asia. Recently, the frequency of MRMP detection has increased in other areas of the world [79-81].
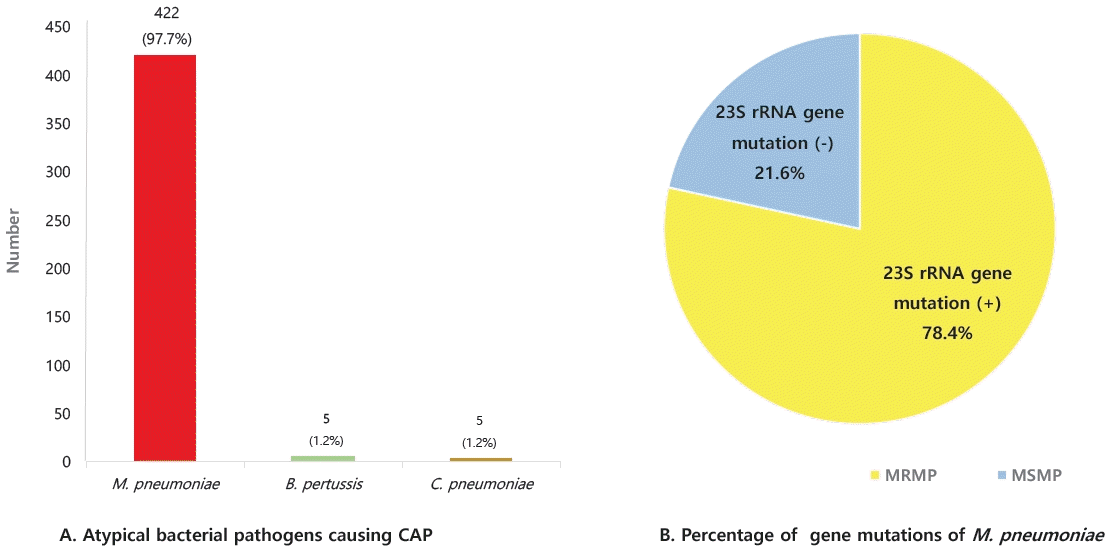
(A) The detected atypical bacterial pathogens in community-acquired pneumonia reported by the Korean Children Community Acquired Pneumonia Study Group.[66] (B) The percentage of gene mutations of Mycoplasma pneumoniae.[73] MRMP, macrolide-resistant M. pneumoniae; MSMP, macrolide-sensitive M. pneumoniae; B. pertussis , Bordetella pertussis ; C. pneumoniae , Chlamydophila pneumoniae.
Mycoplasma and viruses are the most common types of mixed infections. M. pneumoniae is often associated with viral infection, whereas its coinfection with other bacteria is rare. The KoC-CAPS study showed the codetection of virus/M. pneumoniae in 15% and bacteria/M. pneumoniae in 3.7% [66]. It is also known that the rate of coinfection is higher in severe M. Pneumoniae infection than in nonsevere pneumonia [34]. Therefore, severe or poorly treated M. pneumoniae infections should be checked for not only antibiotic resistance but also mixed infection with viruses.
Among the bacteria isolated by culturing, S. aureus was most common (12.8%), followed by S. pneumoniae (9%). This study showed the antibiotic-resistance rate of bacterial pathogens; among a total of 93 cases of S. pneumoniae, 14.1% were resistant to penicillin, 8.7% to cefotaxime, 93.5% to erythromycin, 79.3% to tetracycline, and 1.1% to levofloxacin [66].
As mentioned earlier, the identification of bacteria from the upper respiratory tract does not always determine the causative pathogen of CAP. However, bacterial-virus coinfections may be the causative agents of pneumonia after viral infection. Bacterial pneumonia is often merged in cases in which the ability to remove secretions is reduced due to viral pneumonia, sputum discharge is disrupted due to epithelial damage or normal ciliary motion disorders, and other lung functions are reduced [81]. Respiratory viruses also influence the etiology of pneumonia by altering the bacterial community structure in the upper respiratory tract. Respiratory viruses promote or inhibit the colonization of the lower respiratory tract by certain bacterial species residing in the upper respiratory tract. In particular, S. pneumoniae reportedly has a strong link with viral coinfection, increased carriage, and pneumococcal pneumonia [82].
Conclusion
Pediatric CAP is diagnosed based on clinical and radiologic findings; however, identifying the causative pathogens is important for determining treatment policies. However, pathogen detection in children with CAP is challenging due to difficulty obtaining adequate specimens for microbiological diagnosis. Therefore, pediatricians usually rely on empirical treatment in clinical settings. Obtaining data about the causative pathogens of pediatric CAP will contribute significantly to the surveillance of new pathogens such as SARS-CoV-2. Data on antibiotic resistance will aid the selection of appropriate treatment options, which can reduce the morbidity and mortality of pneumonia in children. In conclusion, it is necessary to continue nationwide monitoring of respiratory CAP pathogens, molecular diagnoses, biological changes of pathogens, and antibiotic resistance.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
The surveillance study of the Korean Childhood Community-Acquired Pneumonia Study Group (KoC-CAPS) was supported by a grant from the Korea Disease Control and Prevention Agency, Republic of Korea (grant number: 4800- 4821-304). This fund provides financial support in the investigation, design of the study, data collection, data analysis, and interpretation of data.
Acknowledgements
The authors would like to thank the members of the Korean Childhood Community Acquired Pneumonia Study Group (KoC-CAPS) of the Korean Academy of Pediatric Allergy and Respiratory Disease
Myongsoon Sung1, Hee Jin Choi1, Mi-Hee Lee2, Ji Young Lee3, Hyo-Bin Kim4, Young Min Ahn5, Ja Kyoung Kim6, Hyoung Young Kim7, Sung-Su Jung7, Minji Kim8, Eun Kyeong Kang9, Eun-Ae Yang10, Soo Jin Lee11, Yang Park12, Ju-Hee Seo13, Eun Lee14, Eun Seok Yang15, Kang Seo Park16, Meeyong Shin17, Hai Lee Chung18, Yoon Young Jang18, Bong Seok Choi19, Hyeona Kim19, Jin-A Jung20, Seung Taek Yu21, Eun Sil Lee22, Jin Tack Kim23, Bong-Seong Kim24, Yoon Ha Hwang25, In-Suk Sol26, Hyeon-Jong Yang27, Man Yong Han28, Hae Young Yew29, Hyoung Min Cho30, Hye-young Kim31, Yeon-Hwa Ahn32, Dong Hyeok Kim33, Kyuhjam Hwang33, Jaeil Yoo33, Sang Oun Jung33 1Department of Pediatrics, Soonchunhyang University Gumi Hospital, Gumi, Korea; 2Department of Pediatrics, Incheon Medical Center, Incheon, Korea; 3Department of Pediatrics, Hallym University Chuncheon Sacred Heart Hospital, Chuncheon, Korea; 4Department of Pediatrics, Inje University Sanggye Paik Hospital, Seoul, Korea; 5Department of Pediatrics, Eulji University Hospital, Seoul, Korea; 6Department of Pediatrics, Kangwon National University School of Medicine, Chuncheon, Korea; 7Department of Pediatrics, Pusan National University Children’s Hospital, Yangsan, Korea; 8Department of Pediatrics, Chungnam National University Sejong Hospital, Chungnam National University College of Medicine, Sejong, Korea; 9Department of Pediatrics, Dongguk University Ilsan Hospital, Goyang, Korea; 10Department of Pediatrics, College of Medicine, The Catholic University of Korea, Daejeon’s St. Mary’s Hospital, Daejeon, Korea; 11Department of Pediatrics, School of Medicine, Eulji University, Daejeon, Korea; 12Department of Pediatrics, Wonkwang University Sanbon Hospital, Wonkwang University College of Medicine, Gunpo, Korea; 13Department of Pediatrics, Dankook University College of Medicine, Cheonan, Korea; 14Department of Pediatrics, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea; 15Department of Pediatrics, Chosun University Hospital, College of Medicine, Chosun University, Gwangju, Korea; 16Department of Pediatrics, Presbyterian Medical Center, Jeonju, Korea; 17Department of Pediatrics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea; 18Department of Pediatrics, Catholic University of Daegu School of Medicine, Daegu, Korea 19Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea; 20Department of Pediatrics, Dong-A University College of Medicine, Busan, Korea; 21Department of Pediatrics, Wonkwang University School of Medicine, Iksan, Korea; 22Department of Pediatrics, Chungnam National University Hospital, Daejeon, Korea; 23Department of Pediatrics, College of Medicine, The Catholic University of Korea, Uijeongbu St. Mary’s Hospital, Uijeongbu, Korea; 24Department of Pediatrics, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea; 25Department of Pediatrics, Busan St. Mary’s Hospital, Busan, Korea; 26Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea; 27Department of Pediatrics, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea; 28Department of Pediatrics, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea; 29Department of Pediatrics, Kogel Hospital, Daejeon, Korea; 30Department of Pediatrics, Kwangju Christian Hospital, Gwangju, Korea; 31Department of Pediatrics, Pusan National University School of Medicine, Busan, Korea; 32Department of Pediatrics, Bundang Jesaeng Hospital, Seongnam, Korea; 33Divison of Bacterial Diseases, Bureau of Infectious Disease Diagnosis Control, Korea Disease Control and Prevention Agency (KDCA), Sejong, Korea