COVID-19 in immunocompromised children and adolescents
Article information
Abstract
Since coronavirus disease 2019 (COVID-19) became a global pandemic, concerns have arisen regarding the risks of COVID-19 in immunocompromised children and adolescents. Here we aimed to evaluate the clinical outcomes and risks of severe COVID-19 in immunocompromised pediatric patients. Previous studies reported that most children and adolescents receiving immunosuppressive medications have clinical presentations and favorable outcomes similar to those of the general pediatric population. Treatments and access to health services should not be interrupted in these populations, and continuous monitoring of the potential impact of variant strains on the risk of immunocompromised pediatric patients is warranted.
Key message
Most immunocompromised children and adolescents are not at increased risk of developing severe coronavirus disease 2019 (COVID-19). COVID-19 outcomes for low- or medium-risk immunocompromised children are favorable, while more serious illness reportedly occurs in high-risk immunocompromised children by underlying disease, its treatments, and other factors. Therefore, the early detection and timely management of severe COVID-19 and treatment of underlying disease are important. Hospitalization and COVID-19 vaccination should be carefully considered.
Graphical abstract
Introduction
Since coronavirus disease 2019 (COVID-19) became a global pandemic, concerns have arisen that immunocompromised patients may be at greater risk of severe infection [1]. Previous studies and national data from different countries demonstrated that children have a considerably milder clinical course and better outcomes than adults when infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2-7]. However, since high-risk children, including immunocompromised patients, could develop severe COVID-19, they are advised to adopt specific precautions without reducing their access to health services [8,9].
In Korea, children and adolescents younger than 19 years of age comprised a small proportion of confirmed COVID-19 cases in the early period of the pandemic, but this proportion increased to 20% of total cases during the omicron variant surge, with a relatively high vaccination rate in adults [10]. Most pediatric patients with asymptomatic or mild COVID-19 have been managed in their own homes according to home care guidelines [11]. However, in immunocompromised children and adolescents, high-risk groups who have the possibility of developing severe COVID-19 should be identified and cared for in the hospital despite limited healthcare resources [4].
While global efforts have increased our understanding of the pathogenesis and treatment of COVID-19, knowledge of clinical outcomes and management in immunocompromised patients is limited, particularly in pediatric patients with inborn errors of immunity and under immunosuppressive medications [9]. Conflicting results have been reported regarding immunocompromised status as a risk factor for developing severe COVID-19 in adults [12-14]. A recent population-based Korean adult study revealed that an immunocompromised status among COVID-19 patients was associated with an increased risk of mortality, whereas malignancy and solid tumors were not [12]. Corticosteroid use and the co-existence of ≥2 causes of the immunosuppression had a significantly increased risk of mortality. This review investigated the clinical outcomes and risks of COVID-19 in immunocompromised pediatric patients to accurately understand and assess the risks in these populations.
Methods
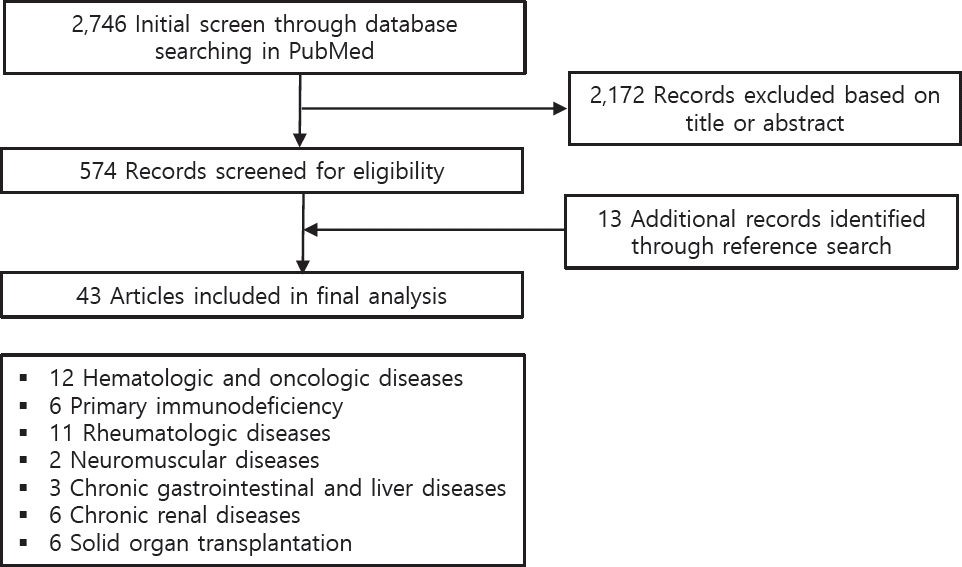
A literature review was performed of the online PubMed database. The entry terms were (“SARS-CoV-2” OR “COVID-19”) AND (“children” OR “pediatric”) AND (“immunocompromised” OR “immunosuppression” OR “autoimmune disease” OR “cancer” or “transplantation” OR “innate immunity” OR “adaptive immunity” OR “immune tolerance” OR “rheumatic diseases” OR “immunosuppressive drugs” OR “biologic drugs” OR “chronic kidney disease” OR “chronic liver disease” OR “hematologic disorder” OR “hematopoietic stem cell transplantation” OR “primary immunodeficiency” OR “chronic gastrointestinal diseases”). Manual searches of the related reference lists were also performed. The results are summarized according to immune mechanisms of disease, including hematologic and oncologic diseases, primary immunodeficiency (PID), rheumatologic diseases, neuromuscular diseases, chronic gastrointestinal and liver diseases, chronic renal diseases, and solid organ transplantation (Fig. 1).
Immune system and clinical aspects of COVID-19 in children and adolescents
Different mechanisms have been described to explain the immune tolerance and milder clinical course in children versus adults [6]. Lower angiotensin-converting enzyme 2 receptor expression, respiratory system characteristics, lower prevalence of comorbidities and risk factors, and effect of school closures and other social distancing measures that could affect resistance to SARS-CoV-2 infection and the different clinical courses between children and adults [1,6].
The innate immunity of children controls SARS-CoV-2 replication and leads to viral clearance without pathogenic inflammatory outcomes [1]. On the other hand, a reduced adaptive immune response in children with milder neutrophil and monocyte/macrophage activation contributes to avoiding hyperinflammation and severe COVID-19. Taken together, the specific mechanisms of immune tolerance to SARS-CoV-2 in children lead to a mild clinical course and better immunopathological consequences of COVID-19 than in adults. In this regard, the hypothesis that patient immunocompromised status might be unimportant or even protective against SARS-CoV-2 infection, as dysregulated and excessive immune responses, seems to drive tissue damage [1].
COVID-19 in children with hematologic and oncologic diseases
Early in the pandemic, children with hemato-oncologic diseases receiving chemotherapy were presumed to be at a higher risk for severe COVID-19 due to their immunocompromised state [15]. However, many case reports and cohort studies from different countries confirmed that the majority of pediatric cancer patients experience relatively mild to moderate symptoms when infected with SARS-CoV-2, although severe COVID-19 and even fatalities may occur in a minority of them.
A systematic review of 45 studies published between April 2020 and October 2021 on 1,003 pediatric cancer patients with SARS-CoV-2 infections revealed that 23.9% of them were asymptomatic, the clinical course of COVID-19 was reportedly mild or moderate in 41.7% of patients, and 11.1% of patients showed severe COVID-19 [15]. Of them, 25 patients (2.5%) eventually died of COVID-19-related conditions. Chemotherapy was postponed in 12.7% of patients, whereas 19% of patients with different underlying malignancies received chemotherapy during the SARS-CoV-2 infection. In some studies, no severe COVID-19 complications were associated with the continuation of chemotherapy. In a review of 226 published cases during the first wave of COVID-19 pandemic, 47% were hospitalized, 10.3% required intensive care, and 4.9% died of COVID-19 [16,17]. In another multinational retrospective registry study of 131 pediatric hemato-oncological patients, the prevalence of severe/critical COVID-19, intensive care unit (ICU) admission, and the mortality was 13%, 11%, and 3%, respectively [18]. Among 1,301 patients reported by the Global Registry COVID-19 in Children Cancer, 67.4% were hospitalized, 19.9% had a severe or critical COVID-19, and 3.8% died of COVID-19 [19]. In these immunocompromised children and adolescents, multisystemic inflammatory syndrome in children (MIS-C) occurring 2–6 weeks after SARS-CoV-2 infection is reported to be less frequent than immunocompetent children [17, 19-21].
In a recent Korean study, Yun et al. reported a nosocomial outbreak of COVID-19 in a daycare unit for pediatric and young adult cancer patients [22]. Among 181 patients exposed to the index case, 3 were infected, but all confirmed cases were asymptomatic or mildly symptomatic. This finding is consistent with previous reports that immunocompromised children and young adults may have mild symptoms and an uncomplicated clinical course but may shed the virus for a prolonged time. These results showed that pediatric patients with hemato-oncologic diseases have more favorable clinical outcomes than adults, probably because of the lower prevalence of other comorbidities and milder COVID-19 symptoms in the younger population [15,23,24].
In contrast, an Algerian study reported a high case fatality rate of 28% (2 of 7) among pediatric cancer patients with SARS-CoV-2 infection and identified differences in the use of critical care resources that might influence the outcome; moreover, these populations could be a vulnerable group in resource-limited settings [24].
Despite a favorable COVID-19 outcome in most pediatric cancer patients, the morbidity rate is reportedly higher than that in the general pediatric population without comorbidities [15]. Therefore, the early identification and timely treatment of severe COVID-19 are important, and the risks of maintaining hematological or oncological treatment should be carefully weighed against the individual benefits. A summary of published studies of children with hemato-oncological diseases and SARS-CoV-2 infection is presented in Table 1.
COVID-19 in children with PID
COVID-19 severity in patients with inborn errors of immunity depends on the underlying defect [1]. An Iranian PID registry showed that eight of 19 patients infected with SARS-CoV-2 died, indicating a 10-fold higher mortality rate among patients with severe combined immunodeficiency and immune dysregulation versus the general population [26]. On the contrary, an Italian registry study of 1,396 pediatric PID patients found a lower incidence of infection (33 of 1,396 [2.36%]) versus the general pediatric population [27]. In another Italian study, only three of 582 pediatric hospital admissions for COVID-19 had PID and none required ICU admission [5]. Another study conducted in Israel presented similar results in which seven of 20 PID patients were asymptomatic with no cases of severe illness and hospitalization, possibly due to high level of awareness, extra precautions, and even self-isolation [28]. A global survey-based study of 32 pediatric PID patients reported that 2 patients died and 9 required intensive care [29]. However, one child with X-linked chronic granulomatous disease had concomitant Burkholderia sepsis and hemophagocytic lymphohistiocytosis (HLH), while the other child had severe gut graft-versus-host disease and developed septic shock and HLH, but the contribution of the SARS-CoV-2 infection to death remains unknown in both cases.
Despite concern of excessive immune activation in autoinflammatory disorders, severe cases have been infrequently reported, possibly due to precautionary measures and protective immunosuppression in these patients [29]. However, a fraction of these patients may develop severe disease requiring intensive care or that even has fatal outcomes. Therefore, further studies of the individual risk of different PID disorders and the potential need for preemptive measures for subsets of PID patients at high risk of developing severe COVID19 are warranted [26].
COVID-19 in immunosuppressed children with rheumatologic diseases (Table 2)
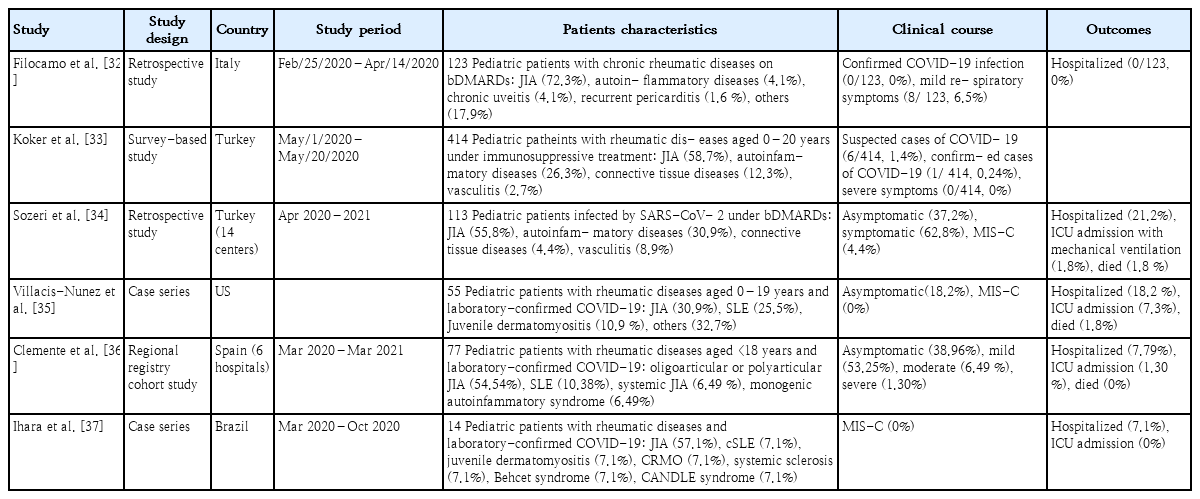
Summary of published studies of pediatric rheumatologic patients with severe acute respiratory syndrome coronavirus 2 infection
Patients with rheumatologic diseases are at increased risk of serious infection due to immunological dysfunction, such as a lower production of specific immunoglobulins, a low complement level, an altered phagocyte response, and the use of immunomodulatory drugs [1]. However, previous population cohort and survey-based studies reported that both adult and pediatric patients with rheumatologic diseases are not at higher risk of severe COVID-19 or worse outcomes versus the general population [30,31].
In an Italian pediatric study conducted early in the pandemic, only 8 of 123 children treated with biological disease-modifying antirheumatic drugs (bDMARDs) had mild respiratory symptoms [33]. No patient required the interruption of ongoing therapy or hospitalization. Consistent with previous studies, a Turkish survey-based study of children with rheumatic diseases treated with immunosuppressive agents reported that only 6 of 414 patients had suspected COVID-19 and none had any severe symptoms [34]. Similar results were reported by a recent Turkish multicenter study of pediatric patients with rheumatic disease receiving bDMARDs [35]. Of 113 patients infected with SARSCoV-2 under bDMARD therapy, 71 (62.8%) had symptomatic COVID-19, 24 (21.2%) required hospitalization, and 2 (1.8%) were admitted to the ICU for mechanical ventilation. Five patients (4.4%) presented with MIS-C, and 2 patients died. No cases of disease flares after COVID-19 have been reported. A case series of pediatric patients with rheumatic diseases and laboratory-confirmed COVID-19 reported that 18.2% of patients were hospitalized and 7.3% required ICU care [36]. The need for hospitalization for COVID-19 was associated with African American race, cardiovascular disease, active rheumatic disease, medium-/high-dose corticosteroids, mycophenolate and rituximab use, and severe immunosuppression.
Previous studies of pediatric rheumatology patients reported low severe COVID-19 and mortality rates, but the literature is less extensive for these diseases and continuous monitoring is needed [30-37]. Furthermore, these patients experienced a lack of medical access and treatment interruptions during the COVID-19 pandemic, which might have led to the progression of their underlying rheumatologic diseases [8,9]. Therefore, it is important to keep the underlying disease under control by maintaining treatment during the pandemic.
COVID-19 in immunosuppressed children with neuromuscular diseases and chronic gastrointestinal, liver, and renal diseases
Patients with neuromuscular diseases tend to be more vulnerable and have higher medical needs than the general population [38]. Respiratory insufficiency, cardiac disease, obesity, and use of immunosuppressive drugs can be risk factors for severe COVID-19 in these patients. In a Spanish study of 153 pediatric patients with neuroimmunological diseases, COVID-19 was diagnosed in 11% (17 of 153), a similar frequency to that of the general pediatric population [39]. No difference was reported in COVID-19 incidence or severity and neurologic relapses between patients treated or untreated with immunosuppressive therapies. All of the infected patients had mild symptoms and recovered completely. Another recent survey conducted from April through May 2020 reported that only 4 of more than 1, 000 patients with spinal muscular atrophy had been diagnosed with COVID-19; of them, only one required hospitalization [38].
Results have been drawn in children with inflammatory bowel diseases (IBDs) or autoimmune liver diseases infected with SARS-CoV-2 [40,41]. Among 102 pediatric IBD centers globally, only 8 children were infected, all with mild symptoms and no need for hospitalization [42]. Although no patient with COVID-19 was reported in Asian countries such as China and South Korea, treatment delays and disease exacerbation were observed in some patients [41]. In an early Italian study, none of the 47 children with liver diseases under immunosuppressive therapy tested positive for COVID-19 [40]. This finding is consistent with those of other studies showing that patients with chronic liver autoimmune diseases are not at increased risk because of immunosuppression [42].
Patients with chronic renal diseases treated with immunosuppressive agents are vulnerable to infections due to malnutrition, uremia, comorbidities, and the use of immunosuppressants [1]. In a recent study performed in Israel of 197 children and 63 young adults with chronic kidney disease treated with immunosuppressants or kidney transplantation, 37% were asymptomatic and the rest were mildly symptomatic [43]. The renal function of the patients remained stable without treatment modification. In a Turkish multicenter study, a total of 46 confirmed pediatric cases of COVID-19 were reported, of which 17 were dialysis patients and 29 were kidney transplant recipients [44]. While most cases were asymptomatic or involved mild respiratory illness, 2 patients had severe COVID-19 and 1 hemodialysis patient with multiple comorbidities died. Children with nephrotic syndrome with or without immunosuppressive therapy are also at increased risk of infection, which may trigger relapse [45].
In a systematic review of 43 pediatric patients with idiopathic nephrotic syndrome and SARS-CoV-2 infection, the clinical course was mild, with a low need for respiratory support for the majority of patients and no deaths among patients in high-income countries [45]. Five patients relapsed but showed a good response to steroid treatment. In contrast, an Indian study of 88 children with nephrotic syndrome and other chronic kidney diseases reported 19.3% had moderate or severe COVID-19; 21.6% had severe complications such as acute kidney injury, encephalopathy, respiratory failure, or shock; and 3.4% died [46]. A global survey-based study including 113 children with COVID-19 treated with immunosuppressive agents for kidney diseases from 30 different countries showed that 78% required no respiratory support and 5% required bi-level positive airway pressure or ventilation [47]. Four patients (3.5%) died, all of whom had associated comorbidities and lived in low-income countries. No significant association was found between immunosuppressive medication type or number and COVID-19 severity. These studies showed that most pediatric patients with chronic kidney diseases under immunosuppressive therapy had a similar incidence of COVID-19 to that of the general population and a mild disease course. However, relatively high mortality and complication rates may occur in developing countries, possibly due to a distinct genetic background or limited access to health services.
Data of pediatric patients who underwent solid organ transplantation showed that immunosuppression was not an additional risk factor for severe or complicated COVID-19, showing that most had mild or moderate disease severity like the general population [42,48-52].
Conclusions
The clinical course and outcome of immunocompromised pediatric patients with COVID-19 are generally favorable, but some caution is still warranted in caring for these patients [9]. Some cases of death have been attributed to concurrent infections or underlying primary disorders, indicating the need for the careful management of secondary infections, disease progression, and immune-related adverse events that may occur without direct relation to COVID-19 [9]. Moreover, atypical manifestations such as HLH or cytopenias are infrequently reported, and evolution of the new variant strains with increased transmissibility and severity may alter clinical outcomes in immunocompromised patients. Hence, we emphasize the need for continuous surveillance and evaluation of the potential impact of COVID-19 on immunocompromised children.
Studies have shown that COVID-19 vaccines (primarily mRNA vaccines) are effective against symptomatic COVID-19 among immunocompromised adult patients but lower than that in immunocompetent adults and varied considerably among subgroups.53,54) In previous pediatric cohort studies, most immunocompromised children and young adults had an appropriate humoral and cellular immune response, side effects were mild, and disease flare rates were low following COVID-19 vaccination [55-57]. This supports the need for a continuous emphasis on COVID-19 vaccination among the immunocompromised pediatric population, consistent with recommendations [55,58,59].
We also suggest that further studies examine the relationship between immunocompromised status and the risk of long COVID in children.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.