Article Contents
| Clin Exp Pediatr > Volume 66(5); 2023 |
|
Abstract
Since coronavirus disease 2019 (COVID-19) became a global pandemic, concerns have arisen regarding the risks of COVID-19 in immunocompromised children and adolescents. Here we aimed to evaluate the clinical outcomes and risks of severe COVID-19 in immunocompromised pediatric patients. Previous studies reported that most children and adolescents receiving immunosuppressive medications have clinical presentations and favorable outcomes similar to those of the general pediatric population. Treatments and access to health services should not be interrupted in these populations, and continuous monitoring of the potential impact of variant strains on the risk of immunocompromised pediatric patients is warranted.
Graphical abstract
Since coronavirus disease 2019 (COVID-19) became a global pandemic, concerns have arisen that immunocompromised patients may be at greater risk of severe infection [1]. Previous studies and national data from different countries demonstrated that children have a considerably milder clinical course and better outcomes than adults when infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2-7]. However, since high-risk children, including immunocompromised patients, could develop severe COVID-19, they are advised to adopt specific precautions without reducing their access to health services [8,9].
In Korea, children and adolescents younger than 19 years of age comprised a small proportion of confirmed COVID-19 cases in the early period of the pandemic, but this proportion increased to 20% of total cases during the omicron variant surge, with a relatively high vaccination rate in adults [10]. Most pediatric patients with asymptomatic or mild COVID-19 have been managed in their own homes according to home care guidelines [11]. However, in immunocompromised children and adolescents, high-risk groups who have the possibility of developing severe COVID-19 should be identified and cared for in the hospital despite limited healthcare resources [4].
While global efforts have increased our understanding of the pathogenesis and treatment of COVID-19, knowledge of clinical outcomes and management in immunocompromised patients is limited, particularly in pediatric patients with inborn errors of immunity and under immunosuppressive medications [9]. Conflicting results have been reported regarding immunocompromised status as a risk factor for developing severe COVID-19 in adults [12-14]. A recent population-based Korean adult study revealed that an immunocompromised status among COVID-19 patients was associated with an increased risk of mortality, whereas malignancy and solid tumors were not [12]. Corticosteroid use and the co-existence of ≥2 causes of the immunosuppression had a significantly increased risk of mortality. This review investigated the clinical outcomes and risks of COVID-19 in immunocompromised pediatric patients to accurately understand and assess the risks in these populations.
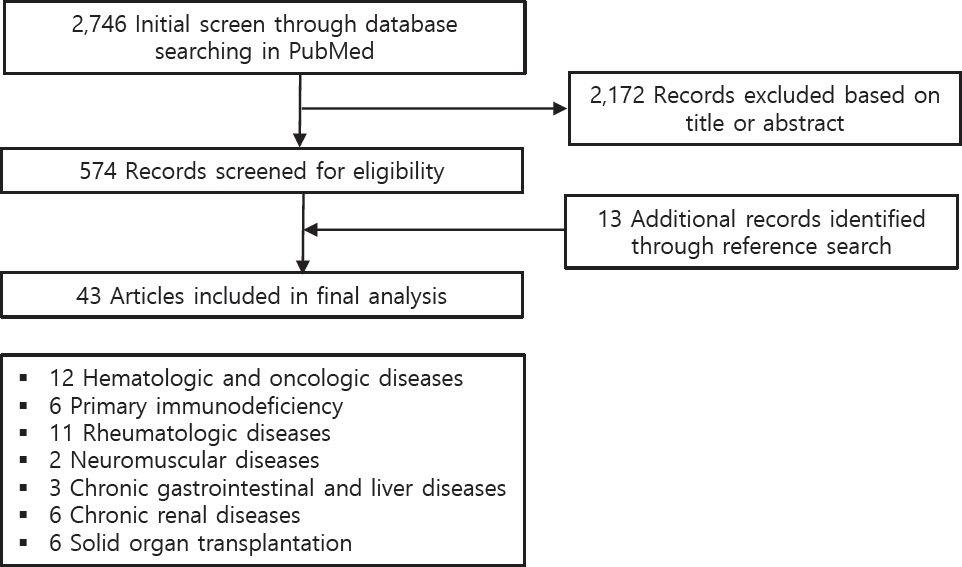
A literature review was performed of the online PubMed database. The entry terms were (“SARS-CoV-2” OR “COVID-19”) AND (“children” OR “pediatric”) AND (“immunocompromised” OR “immunosuppression” OR “autoimmune disease” OR “cancer” or “transplantation” OR “innate immunity” OR “adaptive immunity” OR “immune tolerance” OR “rheumatic diseases” OR “immunosuppressive drugs” OR “biologic drugs” OR “chronic kidney disease” OR “chronic liver disease” OR “hematologic disorder” OR “hematopoietic stem cell transplantation” OR “primary immunodeficiency” OR “chronic gastrointestinal diseases”). Manual searches of the related reference lists were also performed. The results are summarized according to immune mechanisms of disease, including hematologic and oncologic diseases, primary immunodeficiency (PID), rheumatologic diseases, neuromuscular diseases, chronic gastrointestinal and liver diseases, chronic renal diseases, and solid organ transplantation (Fig. 1).
Different mechanisms have been described to explain the immune tolerance and milder clinical course in children versus adults [6]. Lower angiotensin-converting enzyme 2 receptor expression, respiratory system characteristics, lower prevalence of comorbidities and risk factors, and effect of school closures and other social distancing measures that could affect resistance to SARS-CoV-2 infection and the different clinical courses between children and adults [1,6].
The innate immunity of children controls SARS-CoV-2 replication and leads to viral clearance without pathogenic inflammatory outcomes [1]. On the other hand, a reduced adaptive immune response in children with milder neutrophil and monocyte/macrophage activation contributes to avoiding hyperinflammation and severe COVID-19. Taken together, the specific mechanisms of immune tolerance to SARS-CoV-2 in children lead to a mild clinical course and better immunopathological consequences of COVID-19 than in adults. In this regard, the hypothesis that patient immunocompromised status might be unimportant or even protective against SARS-CoV-2 infection, as dysregulated and excessive immune responses, seems to drive tissue damage [1].
Early in the pandemic, children with hemato-oncologic diseases receiving chemotherapy were presumed to be at a higher risk for severe COVID-19 due to their immunocompromised state [15]. However, many case reports and cohort studies from different countries confirmed that the majority of pediatric cancer patients experience relatively mild to moderate symptoms when infected with SARS-CoV-2, although severe COVID-19 and even fatalities may occur in a minority of them.
A systematic review of 45 studies published between April 2020 and October 2021 on 1,003 pediatric cancer patients with SARS-CoV-2 infections revealed that 23.9% of them were asymptomatic, the clinical course of COVID-19 was reportedly mild or moderate in 41.7% of patients, and 11.1% of patients showed severe COVID-19 [15]. Of them, 25 patients (2.5%) eventually died of COVID-19-related conditions. Chemotherapy was postponed in 12.7% of patients, whereas 19% of patients with different underlying malignancies received chemotherapy during the SARS-CoV-2 infection. In some studies, no severe COVID-19 complications were associated with the continuation of chemotherapy. In a review of 226 published cases during the first wave of COVID-19 pandemic, 47% were hospitalized, 10.3% required intensive care, and 4.9% died of COVID-19 [16,17]. In another multinational retrospective registry study of 131 pediatric hemato-oncological patients, the prevalence of severe/critical COVID-19, intensive care unit (ICU) admission, and the mortality was 13%, 11%, and 3%, respectively [18]. Among 1,301 patients reported by the Global Registry COVID-19 in Children Cancer, 67.4% were hospitalized, 19.9% had a severe or critical COVID-19, and 3.8% died of COVID-19 [19]. In these immunocompromised children and adolescents, multisystemic inflammatory syndrome in children (MIS-C) occurring 2–6 weeks after SARS-CoV-2 infection is reported to be less frequent than immunocompetent children [17, 19-21].
In a recent Korean study, Yun et al. reported a nosocomial outbreak of COVID-19 in a daycare unit for pediatric and young adult cancer patients [22]. Among 181 patients exposed to the index case, 3 were infected, but all confirmed cases were asymptomatic or mildly symptomatic. This finding is consistent with previous reports that immunocompromised children and young adults may have mild symptoms and an uncomplicated clinical course but may shed the virus for a prolonged time. These results showed that pediatric patients with hemato-oncologic diseases have more favorable clinical outcomes than adults, probably because of the lower prevalence of other comorbidities and milder COVID-19 symptoms in the younger population [15,23,24].
In contrast, an Algerian study reported a high case fatality rate of 28% (2 of 7) among pediatric cancer patients with SARS-CoV-2 infection and identified differences in the use of critical care resources that might influence the outcome; moreover, these populations could be a vulnerable group in resource-limited settings [24].
Despite a favorable COVID-19 outcome in most pediatric cancer patients, the morbidity rate is reportedly higher than that in the general pediatric population without comorbidities [15]. Therefore, the early identification and timely treatment of severe COVID-19 are important, and the risks of maintaining hematological or oncological treatment should be carefully weighed against the individual benefits. A summary of published studies of children with hemato-oncological diseases and SARS-CoV-2 infection is presented in Table 1.
COVID-19 severity in patients with inborn errors of immunity depends on the underlying defect [1]. An Iranian PID registry showed that eight of 19 patients infected with SARS-CoV-2 died, indicating a 10-fold higher mortality rate among patients with severe combined immunodeficiency and immune dysregulation versus the general population [26]. On the contrary, an Italian registry study of 1,396 pediatric PID patients found a lower incidence of infection (33 of 1,396 [2.36%]) versus the general pediatric population [27]. In another Italian study, only three of 582 pediatric hospital admissions for COVID-19 had PID and none required ICU admission [5]. Another study conducted in Israel presented similar results in which seven of 20 PID patients were asymptomatic with no cases of severe illness and hospitalization, possibly due to high level of awareness, extra precautions, and even self-isolation [28]. A global survey-based study of 32 pediatric PID patients reported that 2 patients died and 9 required intensive care [29]. However, one child with X-linked chronic granulomatous disease had concomitant Burkholderia sepsis and hemophagocytic lymphohistiocytosis (HLH), while the other child had severe gut graft-versus-host disease and developed septic shock and HLH, but the contribution of the SARS-CoV-2 infection to death remains unknown in both cases.
Despite concern of excessive immune activation in autoinflammatory disorders, severe cases have been infrequently reported, possibly due to precautionary measures and protective immunosuppression in these patients [29]. However, a fraction of these patients may develop severe disease requiring intensive care or that even has fatal outcomes. Therefore, further studies of the individual risk of different PID disorders and the potential need for preemptive measures for subsets of PID patients at high risk of developing severe COVID19 are warranted [26].
Patients with rheumatologic diseases are at increased risk of serious infection due to immunological dysfunction, such as a lower production of specific immunoglobulins, a low complement level, an altered phagocyte response, and the use of immunomodulatory drugs [1]. However, previous population cohort and survey-based studies reported that both adult and pediatric patients with rheumatologic diseases are not at higher risk of severe COVID-19 or worse outcomes versus the general population [30,31].
In an Italian pediatric study conducted early in the pandemic, only 8 of 123 children treated with biological disease-modifying antirheumatic drugs (bDMARDs) had mild respiratory symptoms [33]. No patient required the interruption of ongoing therapy or hospitalization. Consistent with previous studies, a Turkish survey-based study of children with rheumatic diseases treated with immunosuppressive agents reported that only 6 of 414 patients had suspected COVID-19 and none had any severe symptoms [34]. Similar results were reported by a recent Turkish multicenter study of pediatric patients with rheumatic disease receiving bDMARDs [35]. Of 113 patients infected with SARSCoV-2 under bDMARD therapy, 71 (62.8%) had symptomatic COVID-19, 24 (21.2%) required hospitalization, and 2 (1.8%) were admitted to the ICU for mechanical ventilation. Five patients (4.4%) presented with MIS-C, and 2 patients died. No cases of disease flares after COVID-19 have been reported. A case series of pediatric patients with rheumatic diseases and laboratory-confirmed COVID-19 reported that 18.2% of patients were hospitalized and 7.3% required ICU care [36]. The need for hospitalization for COVID-19 was associated with African American race, cardiovascular disease, active rheumatic disease, medium-/high-dose corticosteroids, mycophenolate and rituximab use, and severe immunosuppression.
Previous studies of pediatric rheumatology patients reported low severe COVID-19 and mortality rates, but the literature is less extensive for these diseases and continuous monitoring is needed [30-37]. Furthermore, these patients experienced a lack of medical access and treatment interruptions during the COVID-19 pandemic, which might have led to the progression of their underlying rheumatologic diseases [8,9]. Therefore, it is important to keep the underlying disease under control by maintaining treatment during the pandemic.
Patients with neuromuscular diseases tend to be more vulnerable and have higher medical needs than the general population [38]. Respiratory insufficiency, cardiac disease, obesity, and use of immunosuppressive drugs can be risk factors for severe COVID-19 in these patients. In a Spanish study of 153 pediatric patients with neuroimmunological diseases, COVID-19 was diagnosed in 11% (17 of 153), a similar frequency to that of the general pediatric population [39]. No difference was reported in COVID-19 incidence or severity and neurologic relapses between patients treated or untreated with immunosuppressive therapies. All of the infected patients had mild symptoms and recovered completely. Another recent survey conducted from April through May 2020 reported that only 4 of more than 1, 000 patients with spinal muscular atrophy had been diagnosed with COVID-19; of them, only one required hospitalization [38].
Results have been drawn in children with inflammatory bowel diseases (IBDs) or autoimmune liver diseases infected with SARS-CoV-2 [40,41]. Among 102 pediatric IBD centers globally, only 8 children were infected, all with mild symptoms and no need for hospitalization [42]. Although no patient with COVID-19 was reported in Asian countries such as China and South Korea, treatment delays and disease exacerbation were observed in some patients [41]. In an early Italian study, none of the 47 children with liver diseases under immunosuppressive therapy tested positive for COVID-19 [40]. This finding is consistent with those of other studies showing that patients with chronic liver autoimmune diseases are not at increased risk because of immunosuppression [42].
Patients with chronic renal diseases treated with immunosuppressive agents are vulnerable to infections due to malnutrition, uremia, comorbidities, and the use of immunosuppressants [1]. In a recent study performed in Israel of 197 children and 63 young adults with chronic kidney disease treated with immunosuppressants or kidney transplantation, 37% were asymptomatic and the rest were mildly symptomatic [43]. The renal function of the patients remained stable without treatment modification. In a Turkish multicenter study, a total of 46 confirmed pediatric cases of COVID-19 were reported, of which 17 were dialysis patients and 29 were kidney transplant recipients [44]. While most cases were asymptomatic or involved mild respiratory illness, 2 patients had severe COVID-19 and 1 hemodialysis patient with multiple comorbidities died. Children with nephrotic syndrome with or without immunosuppressive therapy are also at increased risk of infection, which may trigger relapse [45].
In a systematic review of 43 pediatric patients with idiopathic nephrotic syndrome and SARS-CoV-2 infection, the clinical course was mild, with a low need for respiratory support for the majority of patients and no deaths among patients in high-income countries [45]. Five patients relapsed but showed a good response to steroid treatment. In contrast, an Indian study of 88 children with nephrotic syndrome and other chronic kidney diseases reported 19.3% had moderate or severe COVID-19; 21.6% had severe complications such as acute kidney injury, encephalopathy, respiratory failure, or shock; and 3.4% died [46]. A global survey-based study including 113 children with COVID-19 treated with immunosuppressive agents for kidney diseases from 30 different countries showed that 78% required no respiratory support and 5% required bi-level positive airway pressure or ventilation [47]. Four patients (3.5%) died, all of whom had associated comorbidities and lived in low-income countries. No significant association was found between immunosuppressive medication type or number and COVID-19 severity. These studies showed that most pediatric patients with chronic kidney diseases under immunosuppressive therapy had a similar incidence of COVID-19 to that of the general population and a mild disease course. However, relatively high mortality and complication rates may occur in developing countries, possibly due to a distinct genetic background or limited access to health services.
The clinical course and outcome of immunocompromised pediatric patients with COVID-19 are generally favorable, but some caution is still warranted in caring for these patients [9]. Some cases of death have been attributed to concurrent infections or underlying primary disorders, indicating the need for the careful management of secondary infections, disease progression, and immune-related adverse events that may occur without direct relation to COVID-19 [9]. Moreover, atypical manifestations such as HLH or cytopenias are infrequently reported, and evolution of the new variant strains with increased transmissibility and severity may alter clinical outcomes in immunocompromised patients. Hence, we emphasize the need for continuous surveillance and evaluation of the potential impact of COVID-19 on immunocompromised children.
Studies have shown that COVID-19 vaccines (primarily mRNA vaccines) are effective against symptomatic COVID-19 among immunocompromised adult patients but lower than that in immunocompetent adults and varied considerably among subgroups.53,54) In previous pediatric cohort studies, most immunocompromised children and young adults had an appropriate humoral and cellular immune response, side effects were mild, and disease flare rates were low following COVID-19 vaccination [55-57]. This supports the need for a continuous emphasis on COVID-19 vaccination among the immunocompromised pediatric population, consistent with recommendations [55,58,59].
We also suggest that further studies examine the relationship between immunocompromised status and the risk of long COVID in children.
Table 1.
Summary of published studies of pediatric hemato-oncologic patients with severe acute respiratory syndrome coronavirus 2 infection
| Study | Study design | Country | Study period | Patients characteristics | Clinical course | Outcomes |
|---|---|---|---|---|---|---|
| Schlage et al. [15] | Systematic review (45 studies) | Multinational: Europe, North America, South America, Africa, Asia | Apr 2020–Oct 2020 (online publication) | 1,003 Pediatric cancer patients aged <18 years | Asymptomatic (23.9%), mild or moderate (41.7 %), severe (11.1%) | Died (2.5%) |
| Wang et al. [16] | Systematic review (33 studies) | Multinational: Italy, US, Spain, Switzland, Austria, Poland, France, Georgia, US, Mexico, Peru, Brazil, India, China, Egypt | Oct/20/2020 (study search) | 226 Pediatric cancer patients aged <18 years | Asymptomatic (30.1%), mild (18.1%), moderate (4.3%), severe (9.6%) | Hospitalized (47%), ICU admission (10.3 %), given oxygen (32.3%), died (4.9 %) |
| Haeusler et al. [18] | Observational cohort study | Multinational: Austria, Italy, Germany, UK, Switzerland, Brazil, Canada, Russia, Israel, Australia | Beginning of the pandemic – Feb/28/2021 | 131 Pediatric patients with cancer or HSCT aged <19 years: leuke- mia (46%), lymphoma (14%), solid tumor (37%), HSCT for primary immunodeficiency (4%) | Asymptomatic (32%), mild (47%), moderate (8%), severe (4%), critical (9%) | ICU admission (11%), died (3%) |
| Mukkada et al. [19] | Cohort study | Multinational (131 institu- tions in 45 countries) | Apr/15/2020– Feb/1/2021 | 1,301 Pediatric patients with can- cer or HSCT aged <19 years: leukemia or lymphoma (49.1%), other hematological malignancies (17.7 %), solid tumor (24.2%), CNS tumor (8.4%), post-HSCT for nonmalignancy (0.5%) | Asymptomatic (35%), mild or moderate (45 %), severe or critical (19.9%) | Hospitalized (67.4%), ICU admission (17.5 %), died (3.8%) |
| Yun et al. [22] | Retrospective study | South Korea | Nov/9/2020– Nov/18/2020 | 181 Pediatric and young adult cancer patients who exposed to the index case | 3 Infected patients were asymptomatic or mildly symptomatic | |
| Arous et al. [25] | Retrospective study | Algeria | - | - | - | Died (28%) |
Table 2.
Summary of published studies of pediatric rheumatologic patients with severe acute respiratory syndrome coronavirus 2 infection
| Study | Study design | Country | Study period | Patients characteristics | Clinical course | Outcomes |
|---|---|---|---|---|---|---|
| Filocamo et al. [32] | Retrospective study | Italy | Feb/25/2020–Apr/14/2020 | 123 Pediatric patients with chronic rheumatic diseases on bDMARDs: JIA (72.3%), autoin- flammatory diseases (4.1%), chronic uveitis (4.1%), recurrent pericarditis (1.6 %), others (17.9%) | Confirmed COVID-19 infection (0/123, 0%), mild re- spiratory symptoms (8/ 123, 6.5%) | Hospitalized (0/123, 0%) |
| Koker et al. [33] | Survey-based study | Turkey | May/1/2020– May/20/2020 | 414 Pediatric patheints with rheumatic dis- eases aged 0–20 years under immunosuppressive treatment: JIA (58.7%), autoinfam- matory diseases (26.3%), connective tissue diseases (12.3%), vasculitis (2.7%) | Suspected cases of COVID- 19 (6/414, 1.4%), confirm- ed cases of COVID-19 (1/ 414, 0.24%), severe symptoms (0/414, 0%) | |
| Sozeri et al. [34] | Retrospective study | Turkey (14 centers) | Apr 2020–2021 | 113 Pediatric patients infected by SARS-CoV- 2 under bDMARDs: JIA (55.8%), autoinfam- matory diseases (30.9%), connective tissue diseases (4.4%), vasculitis (8.9%) | Asymptomatic (37.2%), symptomatic (62.8%), MIS-C (4.4%) | Hospitalized (21.2%), ICU admission with mechanical ventilation (1.8%), died (1.8 %) |
| Villacis-Nunez et al. [35] | Case series | US | 55 Pediatric patients with rheumatic diseases aged 0–19 years and laboratory-confirmed COVID-19: JIA (30.9%), SLE (25.5%), Juvenile dermatomyositis (10.9 %), others (32.7%) | Asymptomatic(18.2%), MIS-C (0%) | Hospitalized (18.2 %), ICU admission (7.3%), died (1.8%) | |
| Clemente et al. [36] | Regional registry cohort study | Spain (6 hospitals) | Mar 2020–Mar 2021 | 77 Pediatric patients with rheumatic diseases aged <18 years and laboratory-confirmed COVID-19: oligoarticular or polyarticular JIA (54.54%), SLE (10.38%), systemic JIA (6.49 %), monogenic autoinfammatory syndrome (6.49%) | Asymptomatic (38.96%), mild (53.25%), moderate (6.49 %), severe (1.30%) | Hospitalized (7.79%), ICU admission (1.30 %), died (0%) |
| Ihara et al. [37] | Case series | Brazil | Mar 2020–Oct 2020 | 14 Pediatric patients with rheumatic diseases and laboratory-confirmed COVID-19: JIA (57.1%), cSLE (7.1%), juvenile dermatomyositis (7.1%), CRMO (7.1%), systemic sclerosis (7.1%), Behcet syndrome (7.1%), CANDLE syndrome (7.1%) | MIS-C (0%) | Hospitalized (7.1%), ICU admission (0%) |
bDMARDs, biological disease-modifying antirheumatic drugs; JIA, juvenile idiopathic arthritis; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MIS-C, multisystemic inflammatory syndrome in children; ICU, intensive care unit; cSLE, childhood-onset systemic lupus erythematosus; CRMO, chronic recurrent multifocal osteomyelitis; CANDLE, chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature.
References
1. Nicastro E, Verdoni L, Bettini LR, Zuin G, Balduzzi A, Montini G, et al. COVID-19 in immunosuppressed children. Front Pediatr 2021;9:629240.



2. Bailey LC, Razzaghi H, Burrows EK, Bunnell HT, Camacho PEF, Christakis DA, et al. Assessment of 135794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr 2021;175:176-84.



3. Bhopal SS, Bagaria J, Olabi B, Bhopal R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health 2021;5:e12-3.



4. Choi JH, Choi SH, Yun KW. Risk factors for severe COVID-19 in children: a systematic review and meta-analysis. J Korean Med Sci 2022;37:e35.




5. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020;4:653-61.



6. Kwak BO, Kim DH. Coronavirus disease 2019: reasons for better clinical course for children compared to adults. Pediatr Infect Vaccine 2021;28:1-6.


7. Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ 2020;370:m3249.



8. Batu ED, Lamot L, Sag E, Ozen S, Uziel Y. How the COVID-19 pandemic has influenced pediatric rheumatology practice: results of a global, cross-sectional, online survey. Semin Arthritis Rheum 2020;50:1262-8.



9. Connelly JA, Chong H, Esbenshade AJ, Frame D, Failing C, Secord E, et al. Impact of COVID-19 on pediatric immunocompromised patients. Pediatr Clin North Am 2021;68:1029-54.



10. Ministry of Health and Welfare. Coronavirus disease-19. Sejong (Korea): Ministry of Health and Welfare, 2022.
11. Korea Disease Control and Prevention Agency. A guideline for COVID-19 self-treatment in children. Cheongju (Korea): Korea Disease Control and Prevention Agency, 2021.
12. Baek MS, Lee MT, Kim WY, Choi JC, Jung SY. COVID-19-related outcomes in immunocompromised patients: a nationwide study in Korea. PLoS One 2021;16:e0257641.



13. Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis 2021;72:340-50.




14. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect 2020;81:e93-5.



15. Schlage S, Lehrnbecher T, Berner R, Simon A, Toepfner N. SARS-CoV-2 in pediatric cancer: a systematic review. Eur J Pediatr 2022;181:1413-27.




16. Wang JG, Zhong ZJ, Mo YF, Wang LC, Chen R. Epidemiological features of coronavirus disease 2019 in children: a meta-analysis. Eur Rev Med Pharmacol Sci 2021;25:1146-57.

17. Meena JP, Kumar Gupta A, Tanwar P, Ram Jat K, Mohan Pandey R, Seth R. Clinical presentations and outcomes of children with cancer and COVID-19: a systematic review. Pediatr Blood Cancer 2021;68:e29005.




18. Haeusler GM, Ammann RA, Carlesse F, Groll AH, Averbuch D, Castagnola E, et al. SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: an analysis of 131 patients. Eur J Cancer 2021;159:78-86.



19. Mukkada S, Bhakta N, Chantada GL, Chen Y, Vedaraju Y, Faughnan L, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol 2021;22:1416-26.


20. Badal S, Thapa Bajgain K, Badal S, Thapa R, Bajgain BB, Santana MJ. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta-analysis. J Clin Virol 2021;135:104715.



21. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020;20:e276-88.



22. Yun KW, Kim YK, Song ES, An HY, Hong KT, Choi JY, et al. Outbreak of COVID-19 among children and young adults in a cancer centre daycare unit. Epidemiol Infect 2022;150:e40.



23. Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open 2021;4:e2111182.



24. Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol 2020;31:1088-9.



25. Arous R, Djillali IS, Rouis NO, Boudiaf H, Amhis W, Ziane H, et al. High mortality of COVID-19 in children with cancer in a single center in Algiers, Algeria. Pediatr Blood Cancer 2021;68:e28898.




26. Delavari S, Abolhassani H, Abolnezhadian F, Babaha F, Iranparast S, Ahanchian H, et al. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J Clin Immunol 2021;41:345-55.




27. Milito C, Lougaris V, Giardino G, Punziano A, Vultaggio A, Carrabba M, et al. Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity. J Allergy Clin Immunol Pract 2021;9:2904-6.e2.



28. Marcus N, Frizinsky S, Hagin D, Ovadia A, Hanna S, Farkash M, et al. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front Immunol 2020;11:614086.



29. Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol 2021;147:520-31.

30. Bourguiba R, Kyheng M, Koné-Paut I, Rouzaud D, Avouac J, Devaux M, et al. COVID-19 infection among patients with autoinflammatory diseases: a study on 117 French patients compared with 1545 from the French RMD COVID-19 cohort: COVIMAI - the French cohort study of SARSCoV-2 infection in patient with systemic autoinflammatory diseases. RMD Open 2022;8:e002063.



31. Michelena X, Borrell H, López-Corbeto M, López-Lasanta M, Moreno E, Pascual-Pastor M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum 2020;50:564-70.



32. Filocamo G, Minoia F, Carbogno S, Costi S, Romano M, Cimaz R. Absence of severe complications from SARS-CoV-2 infection in children with rheumatic diseases treated with biologic drugs. J Rheumatol 2021;48:1343-4.

33. Koker O, Demirkan FG, Kayaalp G, Cakmak F, Tanatar A, Karadag SG, et al. Does immunosuppressive treatment entail an additional risk for children with rheumatic diseases? A survey-based study in the era of COVID-19. Rheumatol Int 2020;40:1613-23.




34. Sozeri B, Ulu K, Kaya-Akça U, Haslak F, Pac-Kisaarslan A, Otar-Yener G, et al. The clinical course of SARS-CoV-2 infection among children with rheumatic disease under biologic therapy: a retrospective and multicenter study. Rheumatol Int 2022;42:469-75.




35. Villacis-Nunez DS, Rostad CA, Rouster-Stevens K, Khosroshahi A, Chandrakasan S, Prahalad S. Outcomes of COVID-19 in a cohort of pediatric patients with rheumatic diseases. Pediatr Rheumatol Online J 2021;19:94.




36. Clemente D, Udaondo C, de Inocencio J, Nieto JC, Del Río PG, Fernández AG, et al. Clinical characteristics and COVID-19 outcomes in a regional cohort of pediatric patients with rheumatic diseases. Pediatr Rheumatol Online J 2021;19:162.




37. Ihara BP, Strabelli CA, Simon JR, Viana VS, Sallum AM, Kozu KT, et al. Laboratory-confirmed pediatric COVID-19 in patients with rheumatic diseases: a case series in a tertiary hospital. Lupus 2021;30:856-60.



38. Stratton AT, Roberts Iii RO, Kupfer O, Carry T, Parsons J, Apkon S. Pediatric neuromuscular disorders: care considerations during the COVID-19 pandemic. J Pediatr Rehabil Med 2020;13:405-14.


39. Olivé-Cirera G, Fonseca E, Cantarín-Extremera V, Vázquez-López M, Jiménez-Legido M, González-Álvarez V, et al. Impact of COVID-19 in immunosuppressed children with neuroimmunologic disorders. Neurol Neuroimmunol Neuroinflamm 2022;9:e1101.



40. Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, et al. Health status of patients with autoimmune liver disease during SARSCoV-2 outbreak in northern Italy. J Hepatol 2020;73:702-5.



41. Turner D, Huang Y, Martín-de-Carpi J, Aloi M, Focht G, Kang B, et al. Coronavirus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2020;70:727-33.



42. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020;26:832-4.



43. Weinbrand-Goichberg J, Ben Shalom E, Rinat C, Choshen S, Tzvi-Behr S, Frishberg Y, et al. COVID-19 in children and young adults with kidney disease: risk factors, clinical features and serological response. J Nephrol 2022;35:121-9.




44. Canpolat N, Yıldırım ZY, Yıldız N, Taşdemir M, Göknar N, Evrengül H, et al. COVID-19 in pediatric patients undergoing chronic dialysis and kidney transplantation. Eur J Pediatr 2022;181:117-23.




45. Morello W, Vianello FA, Proverbio E, Peruzzi L, Pasini A, Montini G. COVID-19 and idiopathic nephrotic syndrome in children: systematic review of the literature and recommendations from a highly affected area. Pediatr Nephrol 2022;37:757-64.




46. Krishnasamy S, Mantan M, Mishra K, Kapoor K, Brijwal M, Kumar M, et al. SARS-CoV-2 infection in children with chronic kidney disease. Pediatr Nephrol 2022;37:849-57.




47. Marlais M, Wlodkowski T, Al-Akash S, Ananin P, Bandi VK, Baudouin V, et al. COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child 2020;106:798-801.



48. Bush R, Johns F, Acharya R, Upadhyay K. Mild COVID-19 in a pediatric renal transplant recipient. Am J Transplant 2020;20:2942-5.




49. Goss MB, Galván NTN, Ruan W, Munoz FM, Brewer ED, O'Mahony CA, et al. The pediatric solid organ transplant experience with COVID-19: an initial multi-center, multi-organ case series. Pediatr Transplant 2021;25:e13868.




50. L'Huillier AG, Danziger-Isakov L, Chaudhuri A, Green M, Michaels MG, K MP-B, et al. SARS-CoV-2 and pediatric solid organ transplantation: current knowns and unknowns. Pediatr Transplant 2021;25:e13986.


51. Marlais M, Wlodkowski T, Vivarelli M, Pape L, Tönshoff B, Schaefer F, et al. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health 2020;4:e17-8.



52. Nicastro E, Di Giorgio A, Zambelli M, Ginammi M, Bravi M, Stroppa P, et al. Impact of the severe acute respiratory syndrome coronavirus 2 outbreak on pediatric liver transplant recipients in Lombardy, Northern Italy. Liver Transpl 2020;26:1359-62.




53. Alexandre RM, Kobayashi T, Suzuki H, Alsuhaibani M, Tofaneto BM, Bariani LM, et al. Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: a systematic literature review and meta-analysis. J Infect 2022;84:297-310.



54. Embi PJ, Levy ME, Naleway AL, Patel P, Gaglani M, Natarajan K, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January-September 2021. Morb Mortal Wkly Rep 2021;70:1553-9.



55. Morgans HA, Bradley T, Flebbe-Rehwaldt L, Selvarangan R, Bagherian A, Barnes AP, et al. Humoral and cellular response to the COVID-19 vaccine in immunocompromised children. Pediatr Res 2022;14:1-6.




56. Sahn B, Lu Y, Hui-Yuen JS, Fishbein J, Gottlieb BS, Eberhard BA, et al. The safety of COVID-19 vaccination in immunocompromised children and young adults with immune-mediated inflammatory disease. Acta Paediatr 2023;112:794-801.




57. Shoji K, Funaki T, Yamada M, Mikami M, Miyake K, Ueno S, et al. Safety of and antibody response to the BNT162b2 COVID-19 vaccine in adolescents and young adults with underlying disease. J Infect Chemother 2023;29:61-6.



58. Korea Disease Control and Prevention Agency. COVID-19 vaccination and incidence in Korea. Press release [Internet]. Cheongju (Korea): Korea Disease Control and Prevention Agency; c2022 [cited 2022 Jan 9]. Available from: https://ncov.kdca.go.kr/?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6405&contSeq=6405&board_id=312&gubun=BDJ.
59. Centers for Disease Control and Prevention. COVID-19 vaccines for people who are moderately or severely immunocompromised [Internet]. Atlanta (GA), Centers for Disease Control and Prevention. c2022;[cited 2022 Jan 9]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


