Virtual reality for pain reduction during intravenous injection in pediatrics: a systematic review and meta-analysis of controlled clinical trials
Article information
Abstract
Background
Intravenous (IV) injections often cause pain, fear, and anxiety in pediatric patients. Virtual reality (VR) is a relatively new intervention that can be used to provide a distraction during or prepare patients for IV injections.
Purpose
To date, no meta-analysis has examined the evidence regarding the effectiveness of VR at reducing pain in pediatric IV injections.
Methods
The PubMed, Web of Science, Scopus, and Cochrane Central Register of Controlled Trials databases were searched for articles published through August 7, 2022. The methodological quality of the studies was measured using the Delphi checklist. The chi-square test and the I2 statistic were used to assess heterogeneity across studies. A summary measure of the mean difference in pain scores between the VR and control groups was obtained using a random effects model. All statistical analyses were set at a significance level of 0.05 using Stata 14.
Results
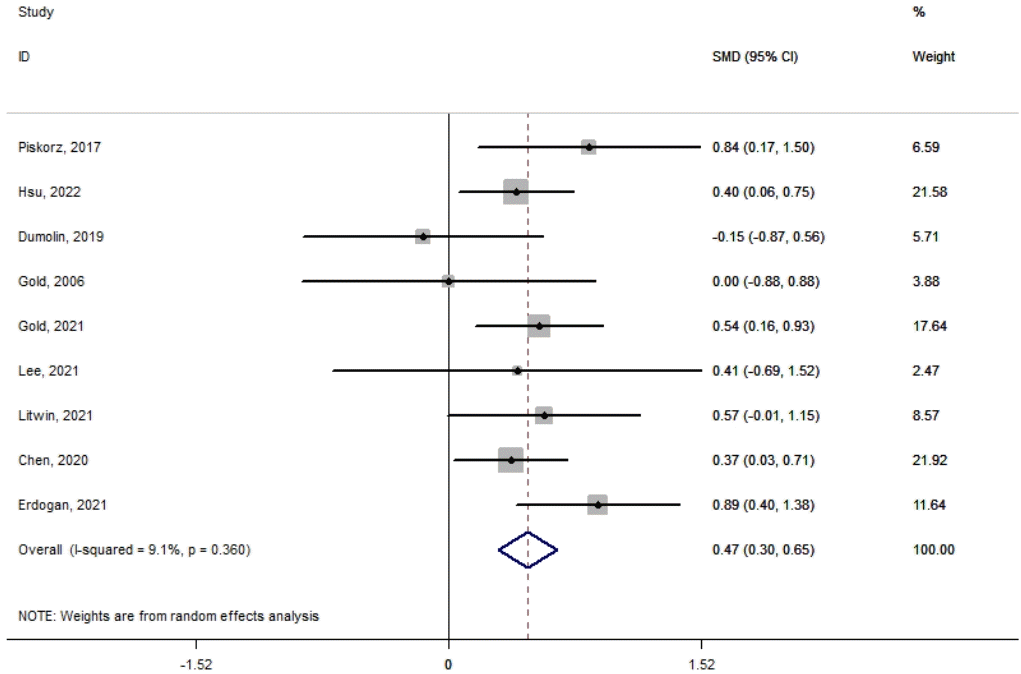
Nine studies were included in this meta-analysis of VR interventions used during IV injections in pediatric patients. The difference in mean pain score between the intervention and control groups showed significant reductions in the VR group (mean difference, 0.47; 95% confidence interval, 0.3–0.65; I2=9.1%). No interstudy heterogeneity was observed.
Conclusion
Our results suggest that VR effectively reduces pain associated with IV injections in pediatric patients. No interstudy heterogeneity was noted among the analyzed studies. The Delphi checklist was used to assess methodological quality.
Key message
Question: This is the first meta-analysis to examine published evidence of the effectiveness of virtual reality at reducing pain during pediatric intravenous injections.
Finding: Our results suggest that virtual reality effectively reduces pain associated with intravenous injections in pediatric patients.
Meaning: These findings suggest the importance of virtual reality in decreasing the pain of intravenous injections among children.
Introduction
While most medical treatments cause anxiety, distress, and associated pain, injections remain the most worrying and disturbing medical procedures for children. In a study of children aged 7–17 years who underwent venipuncture, 52% reported experiencing mild to severe pain [1].
Both drug- and non–drug-based methods can be used to reduce pain during intravascular injections. Despite the therapeutic effects of drugs, the use of medicinal methods is less noticed by patients owing to their side effects, and all types of nonmedicinal methods are used as auxiliary and even alternative treatments owing to the lack of side effects and risks [2]. Several studies have been conducted in different countries using nonpharmacological methods to influence pain quality and amount experienced during intravenous (IV) injections [3-6].
Virtual reality (VR) adds technology to medicine. VR has been defined as a "relatively new tool of human-computer interactions for a human becoming an active participant in a virtual world." [7] VR can be realized using several tools, including personal computer screens, mobile devices, and dedicated rooms. The most often used method for "immersion" into VR is a headmounted visor, which can be connected to a personal computer or linked to a mobile phone [7].
To date, 1 meta-analysis and 3 systematic reviews have examined the effect of VR use in children during medical procedures, but the age ranges differed among the studies; therefore, the number of studies was not considered. The role of VR in changing pain quality experienced by children during IV injections has not been investigated separately; therefore, this systematic review and meta-analysis aimed to determine the effect of VR on pain during IV injections in pediatric patients according to randomized controlled trials (RCTs).
Methods
This systematic review and meta-analysis was conducted according to the PRISMA (preferred items for reporting systematic reviews and meta-analyses) statement [8].
1. Eligibility criteria
This meta-analysis included RCTs that reported the effect of VR on pain reduction during IV injections in pediatric patients aged <18 years. The VR system consisted of a fully immersive computer-generated 3-dimensional (3D) environment displayed in the immersive stereoscopic view of a head-mounted display (HMD). We excluded observational studies, meta-analyses, reviews, case reports and series, and letters to the editor.
2. Information sources and search
The PubMed, Web of Science, Scopus, and Cochrane Central Register of Controlled Trials databases were searched for articles published from their inception through August 7, 2022. The reference lists of the included RCTs were manually searched to identify any other relevant studies. No language restrictions were imposed. The search terms were "virtual reality OR virtual reality exposure therapy" and "pain OR ache" and "boy, child, childhood, girl, infant, kid, pediatrics, preschool, school, toddler, high school, juvenile, minor, prepubescent, prepuberty, pubescent, puberty, teen, teenager, under aged, youth, OR adolescent" and "intravenous injection, intravenous insertion, intravenous placement, OR venipuncture" and "clinical trial, controlled trial, OR randomized controlled trial."
3. Study selection
EndNote software was used to include the search findings and remove duplicate references. EJ and SB independently screened the titles and abstracts and then evaluated the identified studies based on the exclusion and inclusion criteria. Any disagreements were discussed until consensus was reached.
4. Data extraction
Two researchers (EJ and AS) independently extracted data from an electronic data sheet. Any disagreements between the 2 authors were resolved by consensus. We extracted information from the datasheet, including the first author's name (year of publication), country, assessment instruments for pain, children’s ages, sample sizes of the intervention and control groups, virtual equipment, and control program.
5. Methodological quality
The methodological quality of the studies was assessed using the Delphi checklist [9], items of which include standard randomization, concealed allocation of intervention, patient-blinded, care provider-blinded, outcome assessor-blinded, 2 groups similar at baseline, well-defined eligibility criteria, variability of the outcome presented, and intention-to-treat analysis. In this study, the items of the patient-blinded, care provider-blinded, and outcome assessor-blinded were deleted due to the nature of the VR method. Therefore, based on the checklist, we considered a maximum score of 6 points for each study.
6. Heterogeneity and publication biases
The chi-square test [10] and the I2 statistic were applied to measure heterogeneity across studies [11].
7. Summary measures
A summary of the mean differences in pain scores between the VR and control groups was obtained using a random effects model [12]. All statistical analyses were set at a significance level of 0.05 using Stata 14 (StataCorp, College Station, TX, USA).
Results
1. Description of studies
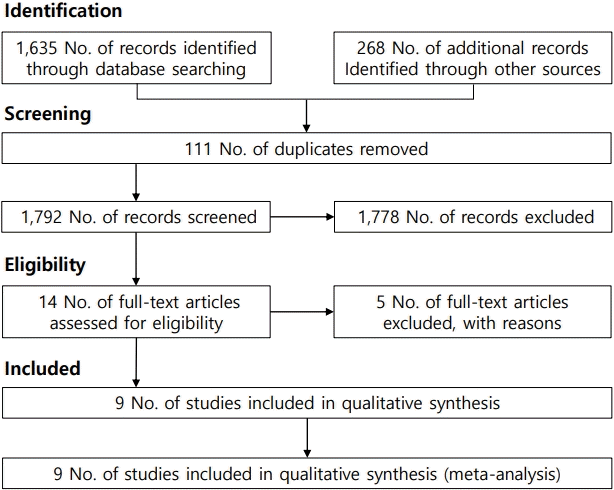
Table 1 summarizes the characteristics of the studies included in the meta-analysis. Based on the selection process (Fig. 1), a total of 9 studies [3-6,13-17] examined VR interventions during IV injection among pediatric patients. Of them, 3 were conducted in Korea, 2 in Taiwan, 2 in Canada, 1 in Poland, and 1 in Turkey. To assess pain, 3 studies used the visual analogue scale, 3 used the Wong-Baker FACES Pain Scale, 1 used the numerical rating scale, 1 used the Faces Pain Scale-Revised, 1 one used the FLACC (faces, legs, activity, cry, consolability) scale. The meta-analysis included 646 participants (324 in the control group, 322 in the intervention group). These studies were conducted between 2006 and 2022 (Table 1). All included studies were published in English.
2. Effects of exposure
Fig. 2 shows the VR interventions used during IV injections in pediatric patients. The meta-analysis of the difference in mean pain score between the intervention and control groups showed significant reductions in the VR group (mean difference, 0.47; 95% confidence interval, 0.3–0.65; I2=9.1%). No heterogeneity was observed among the included studies.
3. Publication bias
Begg test did not report publication bias among the trials. The P value for Begg regression was 0.881.
4. Study quality
The quality of studies based on the Delphi checklist is presented in Table 1.
Discussion
To the best of our knowledge, this is the first meta-analysis to report the effectiveness of VR during pediatric IV injections based on observational RCTs. Our findings indicated that VR is a feasible distraction method for pediatric patients during IV injections. There was no heterogeneity among the examined studies.
In VR, users interact in a computer-simulated 3D environment. VR technology provides multisensory information that helps a person become fully immersed in a simulated world. Users wear HMD helmets that provide stereo images and create a sense of space and depth. The motion detector in the HMD helmet measured the position of the head and adjusted the visual image accordingly. Consequently, users feel that they can look at and move around in the simulated environment. Headphones provide sounds that further help one immerse oneself in the virtual world [18,19], thus distracting the mind from the pain [20].
Triberti et al. [21] investigated the psychological factors affecting pain reduction based on VR and showed that distraction had the greatest effect on pain reduction after reviewing 11 studies. Moreover, the feeling of being in another environment effectively creates distraction.
Although several systematic reviews and meta-analyses have examined the effect of using VR on various types of pain in different age groups, the present study is the first to examine the ability of VR to reduce pain during IV injections in pediatric patients.
A systematic review and meta-analysis by Eijlers et al. [18] of 17 studies examining the effect of VR on pain and anxiety in children during medical procedures such as venous blood sampling, dental procedures, burns, and chemotherapy reported that VR reduces children's pain and anxiety during the aforementioned procedures; however, in this study, a greater number of studies examined the effect of VR on burns [22].
Similar to our study, a systematic review and meta-analysis by Chan et al. [23] aimed to determine the effect of VR on acute pain after reviewing 16 articles showing that VR effectively reduced acute pain. However, unlike our study, this study had high clinical and statistical interstudy heterogeneity; therefore, the findings suggest that VR is more effective in pediatric than adult patients.
Subgroup analyses are needed to clarify the association between VR and pain reduction in pediatric patients during IV injection in terms of VR type, VR duration, and child's sex. However, pain reduction through the VR experience may differ among ages and diseases, representing a limitation of this study.
In conclusion, pain management during medical care is a basic human right that affects patient satisfaction. Moreover, effective acute pain management contributes to improved outcomes and patient satisfaction. Considering the ability of VR to reduce pain in pediatric patients during IV injections, this tool may be used to reduce pain. This meta-analysis identified no heterogeneity among studies of the effectiveness of VR in reducing IV injection pain in pediatric patients.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This meta-analysis was supported by Hamadan University of Medical Sciences with Ethical Code IR.UMSHA. REC.1401.533.
Author contribution
Data curation: EJ, SB, AS; Formal analysis: EJ; Funding acquisition: SB; Methodology: EJ, MB, MR; Project administration: SB; Visualization: SB, EJ, MB; Writing - original draft: All authors; Writing - review & editing: All authors