Article Contents
| Clin Exp Pediatr > Volume 66(12); 2023 |
|
Abstract
Background
Intravenous (IV) injections often cause pain, fear, and anxiety in pediatric patients. Virtual reality (VR) is a relatively new intervention that can be used to provide a distraction during or prepare patients for IV injections.
Purpose
To date, no meta-analysis has examined the evidence regarding the effectiveness of VR at reducing pain in pediatric IV injections.
Methods
The PubMed, Web of Science, Scopus, and Cochrane Central Register of Controlled Trials databases were searched for articles published through August 7, 2022. The methodological quality of the studies was measured using the Delphi checklist. The chi-square test and the I2 statistic were used to assess heterogeneity across studies. A summary measure of the mean difference in pain scores between the VR and control groups was obtained using a random effects model. All statistical analyses were set at a significance level of 0.05 using Stata 14.
Results
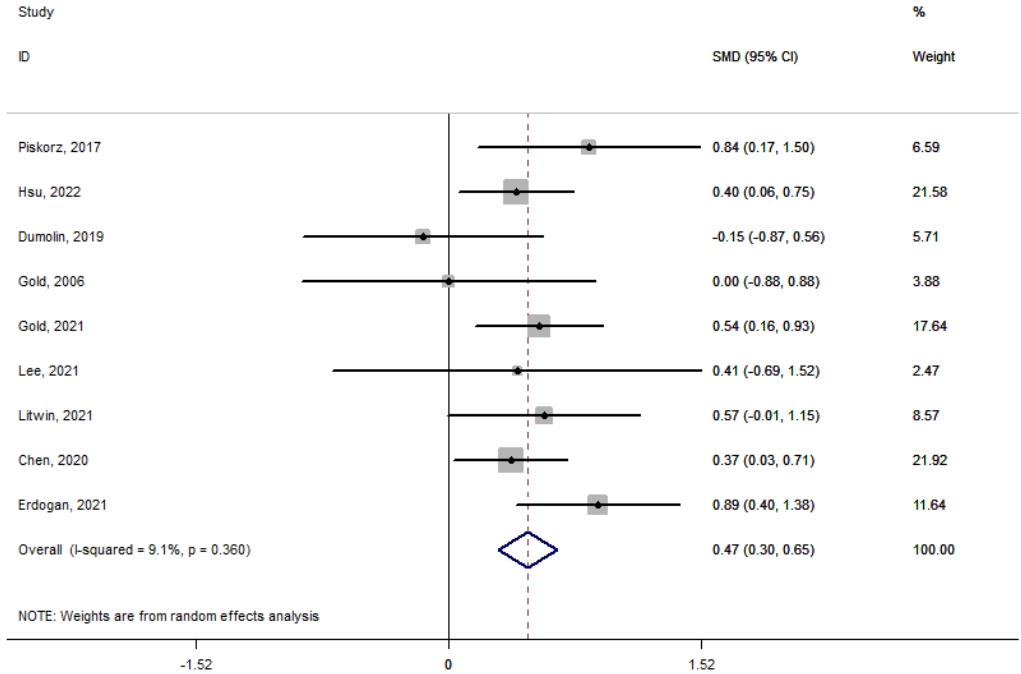
Nine studies were included in this meta-analysis of VR interventions used during IV injections in pediatric patients. The difference in mean pain score between the intervention and control groups showed significant reductions in the VR group (mean difference, 0.47; 95% confidence interval, 0.3ŌĆō0.65; I2=9.1%). No interstudy heterogeneity was observed.
While most medical treatments cause anxiety, distress, and associated pain, injections remain the most worrying and disturbing medical procedures for children. In a study of children aged 7ŌĆō17 years who underwent venipuncture, 52% reported experiencing mild to severe pain [1].
Both drug- and nonŌĆōdrug-based methods can be used to reduce pain during intravascular injections. Despite the therapeutic effects of drugs, the use of medicinal methods is less noticed by patients owing to their side effects, and all types of nonmedicinal methods are used as auxiliary and even alternative treatments owing to the lack of side effects and risks [2]. Several studies have been conducted in different countries using nonpharmacological methods to influence pain quality and amount experienced during intravenous (IV) injections [3-6].
Virtual reality (VR) adds technology to medicine. VR has been defined as a "relatively new tool of human-computer interactions for a human becoming an active participant in a virtual world." [7] VR can be realized using several tools, including personal computer screens, mobile devices, and dedicated rooms. The most often used method for "immersion" into VR is a headmounted visor, which can be connected to a personal computer or linked to a mobile phone [7].
To date, 1 meta-analysis and 3 systematic reviews have examined the effect of VR use in children during medical procedures, but the age ranges differed among the studies; therefore, the number of studies was not considered. The role of VR in changing pain quality experienced by children during IV injections has not been investigated separately; therefore, this systematic review and meta-analysis aimed to determine the effect of VR on pain during IV injections in pediatric patients according to randomized controlled trials (RCTs).
This systematic review and meta-analysis was conducted according to the PRISMA (preferred items for reporting systematic reviews and meta-analyses) statement [8].
This meta-analysis included RCTs that reported the effect of VR on pain reduction during IV injections in pediatric patients aged <18 years. The VR system consisted of a fully immersive computer-generated 3-dimensional (3D) environment displayed in the immersive stereoscopic view of a head-mounted display (HMD). We excluded observational studies, meta-analyses, reviews, case reports and series, and letters to the editor.
The PubMed, Web of Science, Scopus, and Cochrane Central Register of Controlled Trials databases were searched for articles published from their inception through August 7, 2022. The reference lists of the included RCTs were manually searched to identify any other relevant studies. No language restrictions were imposed. The search terms were "virtual reality OR virtual reality exposure therapy" and "pain OR ache" and "boy, child, childhood, girl, infant, kid, pediatrics, preschool, school, toddler, high school, juvenile, minor, prepubescent, prepuberty, pubescent, puberty, teen, teenager, under aged, youth, OR adolescent" and "intravenous injection, intravenous insertion, intravenous placement, OR venipuncture" and "clinical trial, controlled trial, OR randomized controlled trial."
EndNote software was used to include the search findings and remove duplicate references. EJ and SB independently screened the titles and abstracts and then evaluated the identified studies based on the exclusion and inclusion criteria. Any disagreements were discussed until consensus was reached.
Two researchers (EJ and AS) independently extracted data from an electronic data sheet. Any disagreements between the 2 authors were resolved by consensus. We extracted information from the datasheet, including the first author's name (year of publication), country, assessment instruments for pain, childrenŌĆÖs ages, sample sizes of the intervention and control groups, virtual equipment, and control program.
The methodological quality of the studies was assessed using the Delphi checklist [9], items of which include standard randomization, concealed allocation of intervention, patient-blinded, care provider-blinded, outcome assessor-blinded, 2 groups similar at baseline, well-defined eligibility criteria, variability of the outcome presented, and intention-to-treat analysis. In this study, the items of the patient-blinded, care provider-blinded, and outcome assessor-blinded were deleted due to the nature of the VR method. Therefore, based on the checklist, we considered a maximum score of 6 points for each study.
A summary of the mean differences in pain scores between the VR and control groups was obtained using a random effects model [12]. All statistical analyses were set at a significance level of 0.05 using Stata 14 (StataCorp, College Station, TX, USA).
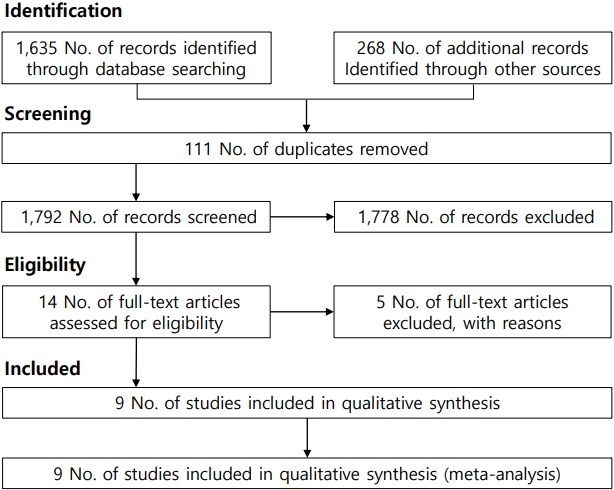
Table 1 summarizes the characteristics of the studies included in the meta-analysis. Based on the selection process (Fig. 1), a total of 9 studies [3-6,13-17] examined VR interventions during IV injection among pediatric patients. Of them, 3 were conducted in Korea, 2 in Taiwan, 2 in Canada, 1 in Poland, and 1 in Turkey. To assess pain, 3 studies used the visual analogue scale, 3 used the Wong-Baker FACES Pain Scale, 1 used the numerical rating scale, 1 used the Faces Pain Scale-Revised, 1 one used the FLACC (faces, legs, activity, cry, consolability) scale. The meta-analysis included 646 participants (324 in the control group, 322 in the intervention group). These studies were conducted between 2006 and 2022 (Table 1). All included studies were published in English.
Fig. 2 shows the VR interventions used during IV injections in pediatric patients. The meta-analysis of the difference in mean pain score between the intervention and control groups showed significant reductions in the VR group (mean difference, 0.47; 95% confidence interval, 0.3ŌĆō0.65; I2=9.1%). No heterogeneity was observed among the included studies.
To the best of our knowledge, this is the first meta-analysis to report the effectiveness of VR during pediatric IV injections based on observational RCTs. Our findings indicated that VR is a feasible distraction method for pediatric patients during IV injections. There was no heterogeneity among the examined studies.
In VR, users interact in a computer-simulated 3D environment. VR technology provides multisensory information that helps a person become fully immersed in a simulated world. Users wear HMD helmets that provide stereo images and create a sense of space and depth. The motion detector in the HMD helmet measured the position of the head and adjusted the visual image accordingly. Consequently, users feel that they can look at and move around in the simulated environment. Headphones provide sounds that further help one immerse oneself in the virtual world [18,19], thus distracting the mind from the pain [20].
Triberti et al. [21] investigated the psychological factors affecting pain reduction based on VR and showed that distraction had the greatest effect on pain reduction after reviewing 11 studies. Moreover, the feeling of being in another environment effectively creates distraction.
Although several systematic reviews and meta-analyses have examined the effect of using VR on various types of pain in different age groups, the present study is the first to examine the ability of VR to reduce pain during IV injections in pediatric patients.
A systematic review and meta-analysis by Eijlers et al. [18] of 17 studies examining the effect of VR on pain and anxiety in children during medical procedures such as venous blood sampling, dental procedures, burns, and chemotherapy reported that VR reduces children's pain and anxiety during the aforementioned procedures; however, in this study, a greater number of studies examined the effect of VR on burns [22].
Similar to our study, a systematic review and meta-analysis by Chan et al. [23] aimed to determine the effect of VR on acute pain after reviewing 16 articles showing that VR effectively reduced acute pain. However, unlike our study, this study had high clinical and statistical interstudy heterogeneity; therefore, the findings suggest that VR is more effective in pediatric than adult patients.
Subgroup analyses are needed to clarify the association between VR and pain reduction in pediatric patients during IV injection in terms of VR type, VR duration, and child's sex. However, pain reduction through the VR experience may differ among ages and diseases, representing a limitation of this study.
In conclusion, pain management during medical care is a basic human right that affects patient satisfaction. Moreover, effective acute pain management contributes to improved outcomes and patient satisfaction. Considering the ability of VR to reduce pain in pediatric patients during IV injections, this tool may be used to reduce pain. This meta-analysis identified no heterogeneity among studies of the effectiveness of VR in reducing IV injection pain in pediatric patients.
Footnotes
Fig.┬Ā2.
Meta-analysis of the randomized controlled trials of the virtual reality interventions during intravenous injections among pediatric patients. SMD, standardized mean difference; CI, confidence interval.
Table┬Ā1.
Characteristics of the studies included in the meta-analysis
| Study | Country | Sample size, intervention/control | VR equipment | Control program | Scale for measuring pain | Age (yr), range | Quality |
|---|---|---|---|---|---|---|---|
| Piskorz and Czub [6] (2017) | Poland | 19/19 | Oculus Rift DK2 | Standard care | Visual analogue scale | 7ŌĆō17 | 1 |
| Hsu et al. [4] (2022) | Taiwan | 69/65 | The VR headset HTC Vive | Educational photo book about intravenous place┬Ł ment before intravenous placement | Wong┬Łbaker faces | 6ŌĆō12 | 5 |
| Gold et al. [3] (2006) | Korea | 30/33 | Street Luge | Standard care | Wong┬Łbaker faces | 7ŌĆō12 | 5 |
| Dumoulin et al. [14] (2019) | Canada | 20/15 | An immersive game developed by the UQO Cyberpsychology Lab using Virtools 4 | Standard care | Visual analogue scale | 8ŌĆō17 | 5 |
| Gold et al. [16] (2021) | Korea | 53/54 | Two mobile┬Łbased VR head┬Ł mounted displays | Standard care | Faces pain scale┬Łrevised | 12.8ŌĆō16.9 | 2 |
| Lee et al. [5] (2021) | Korea | 5/9 | VR animation through the dome screen within 1 minute after intravenous replacement | Without virtual reality dis┬Łtraction | FLACC | 2ŌĆō6 | 3 |
| Litwin et al. [17] (2021) | Canada | 24/24 | Stereoscopic display mounted on a lightweight wireless HMD | Children were given a tablet playing a video of fish and sea turtles swimming in the ocean | Numbering rating scale | 8ŌĆō17 | 5 |
| Chen et al. [13] (2020) | Taiwan | 68/68 | Roller coasters, space explora┬Ł tion, a wildlife park, and travel destinations | Routine intravenous injec┬Ł tion procedure | Wong┬Łbaker faces | 7ŌĆō12 | 5 |
| Erdogan et al. [15] (2021) | Turkey | 34/37 | A smartphone, VR glasses and a headset | No intervention | Visual analogue scale | 7ŌĆō12 | 2 |
References
1. McLenon J, Rogers MAM. The fear of needles: a systematic review and meta-analysis. J Adv Nurs 2019;75:30ŌĆō42.



2. Basak T, Duman S, Demirtas A. Distraction-based relief of pain associated with peripheral intravenous catheterisation in adults: a randomised controlled trial. J Clin Nurs 2020;29:770ŌĆō7.



3. Gold JI, Kim SH, Kant AJ, Joseph MH, Rizzo AS. Effectiveness of virtual reality for pediatric pain distraction during i.v. placement. Cyberpsychol Behav 2006;9:207ŌĆō12.


4. Hsu MF, Whu YW, Lin IC, Liu CY, Lai FC, Liu PC, et al. Effectiveness of virtual reality interactive play for children during intravenous placement: a randomized controlled trial. Asian Nurs Res (Korean Soc Nurs Sci) 2022;16:87ŌĆō93.


5. Lee HN, Bae W, Park JW, Jung JY, Hwang S, Kim DK, et al. Virtual reality environment using a dome screen for procedural pain in young children during intravenous placement: a pilot randomized controlled trial. PLoS One 2021;16:e0256489.



6. Piskorz J, Czub M. Effectiveness of a virtual reality intervention to minimize pediatric stress and pain intensity during venipuncture. J Spec Pediatr Nurs 2018 Jan;23(1). doi: 10.1111/jspn.12201. [Epub].


7. Iannicelli AM, Vito D, Dodaro CA, De Matteo P, Nocerino R, Sepe A, et al. Does virtual reality reduce pain in pediatric patients? A systematic review. Ital J Pediatr 2019;45:171.




8. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1.




9. Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235ŌĆō41.

10. Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0. 2 [Internet]. The Cochrane Collaboration, 2009 [updated 2009 Sep; cited 2023 May 20]. Available from: https://handbook-5-1.cochrane.org/chapter_6/6_searching_for_studies.htm.
11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557ŌĆō60.



13. Chen YJ, Cheng SF, Lee PC, Lai CH, Hou IC, Chen CW. Distraction using virtual reality for children during intravenous injections in an emergency department: a randomised trial. J Clin Nurs 2020;29:503ŌĆō10.



14. Dumoulin S, Bouchard S, Ellis J, Lavoie KL, V├®zina MP, Charbonneau P, et al. A Randomized controlled trial on the use of virtual reality for needle-related procedures in children and adolescents in the emergency department. Games Health J 2019;8:285ŌĆō93.


15. Erdogan B, Aytekin Ozdemir A. The effect of three different methods on venipuncture pain and anxiety in children: distraction cards, virtual reality, and Buzzy┬« (randomized controlled trial). J Pediatr Nurs 2021;58:e54ŌĆō62.


16. Gold JI, SooHoo M, Laikin AM, Lane AS, Klein MJ. Effect of an immersive virtual reality intervention on pain and anxiety associated with peripheral intravenous catheter placement in the pediatric setting: a randomized clinical trial. JAMA Netw Open 2021;4:e2122569.



17. Litwin SP, Nguyen C, Hundert A, Stuart S, Liu D, Maguire B, et al. Virtual reality to reduce procedural pain during IV insertion in the pediatric emergency department: a pilot randomized controlled trial. Clin J Pain 2021;37:94ŌĆō101.

18. Eijlers R, Utens EMWJ, Staals LM, de Nijs PFA, Berghmans JM, Wijnen RMH, et al. Systematic review and meta-analysis of virtual reality in pediatrics: effects on pain and anxiety. Anesth Analg 2019;129:1344ŌĆō53.



19. Aliakbari M, Alipour A, Ebrahimimoghadam H, Fekraty M. The effect of virtual reality (VR) on psychological disorders in cancer cases. Mil Caring Sci 2017;4:49ŌĆō57.
20. Ebrahimiyan A, Rahmany-Bilandi R. The effects of virtual reality on pain stages of labor: a systematic review of clinical trials. J Isfahan Med Sch 2022;40:474ŌĆō80.
21. Triberti S, Repetto C, Riva G. Psychological factors influencing the effectiveness of virtual reality-based analgesia: a systematic review. Cyberpsychol Behav Soc Netw 2014;17:335ŌĆō45.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


