Natural course of IgE-mediated food allergy in children
Article information
Abstract
The prevalence of food allergy and food-induced anaphylaxis in children is increasing worldwide. Cow’s milk, hen’s eggs, and wheat allergies in young children have a more favorable prognosis with a relatively early outgrow, while allergies to peanuts, tree nuts, and seafood are more likely to be persistent. Although our understanding of the mechanism underlying the resolution of food allergy is incomplete, the roles of dendritic cells, regulatory T cells, and regulatory B cells are important. Many past studies on the natural course of food allergy were retrospective analyses of specific study groups, but large-scale population-based prospective studies are now being published. This review summarizes recent studies of the natural course of cow’s milk, hen’s eggs, wheat, peanuts, tree nuts, soy, sesame, and seafood allergies. The potential factors affecting the natural course of food allergy include symptom severity on ingestion, age at diagnosis, allergic comorbidities, skin prick test reaction size or serum food-specific immunoglobulin (Ig) E levels, changes in sensitization degree, IgE epitope specificity, ratio of food-specific IgE to IgG4, food-specific IgA levels, component-resolved diagnostic profile, diet, gut microbiome, and interventions such as immunotherapy. Since food allergy places a significant burden on patients and their caregivers in daily life, clinicians should be able to provide relevant knowledge on the natural course of food allergy, appropriately evaluate its resolution, and offer therapeutic options whenever possible.
Key message
· Dendritic, regulatory T, and regulatory B cells significantly contribute to the natural course of food allergy.
· Cow’s milk and hen’s egg allergies tend to resolve in earlier childhood but recent studies show that 50% of patients still persist into school age.
· The potential factors affecting the natural course of food allergy are age at diagnosis, symptom severity, sensitization status and its change rate, and external factors such as diet and interventions.
· There is a considerable possibility of food allergy outgrow if specific IgE levels are 2–5 kUA/L or less, but other factors such as age and recent symptoms should be considered together.
· With a clear understanding of the natural course of food allergy, pediatricians can provide appropriate assessment and interventions to our patients, and consequently can help patients overcome their food allergy and improve the social safety net.
Introduction
The prevalence of immunoglobulin (Ig) E–mediated food allergy in children has increased remarkably worldwide in recent decades, placing a substantial burden on patients, their caregivers, and health care systems. The results of food allergy epidemiologic studies vary by study method, such as whether the diagnosis is based on an oral food challenge (OFC), a clinician’s history taking, or self-reported questionnaires, but it is apparent that the prevalence of IgE-mediated food allergy in children is increasing despite variations in research designs [1]. Large-scale population-based studies in Australia reported that the prevalence of food allergy exceeded 10% in infants and was 4%–5% in older children [2,3]. According to a cross-sectional population-based survey in the United States, the prevalence of IgE-mediated food allergy in children was 7.6%.4) In a Korean birth cohort study, the prevalence of immediate-type food allergy was 5.3% among infants [5]. Potentially life-threatening food-induced anaphylaxis is of greater importance, especially in younger individuals, in whom a sharp increase has been observed compared to older individuals. The annual admission rates due to foodinduced anaphylaxis in Australia increased 9-fold between 1998–1999 and 2018–2019, with the highest rates in those younger than 1 year of age [6]. According to a national big data analysis in Korea, the prevalence of all-cause anaphylaxis increased 1.7-fold from 2010 to 2014 and was greatest in the 0–2 years age group, in which food-induced anaphylaxis was most frequent [7].
Allergies to peanuts, tree nuts, and seafood tend to persist, whereas those to cow’s milk, hen’s eggs, wheat, and soy are typically outgrown during the preschool years. However, some studies suggested that the resolution timing of these food allergies may now be delayed compared with past decades [8,9]. Food allergy patients and/or their caregivers often experience a significant decrease in quality of life, especially if they experience severe symptoms, have multiple food allergies, or have allergies to staple foods that are more difficult to avoid, such as cow’s milk, hen’s eggs, or wheat [10,11]. Therefore, it is important for clinicians to equip themselves with current knowledge about the natural course of food allergy to guide elimination diets as well as to evaluate food allergy resolution at appropriate timing during follow-up. This review discusses the natural course of major IgE-mediated food allergies in children.
Mechanisms related to resolution of IgE-mediated food allergy
Our understanding remains poor about why some food allergies outgrow earlier versus persist longer. Tolerance to food allergens is driven mainly by antigen-presenting cells within the gut lamina propria by the promotion of T-cell differentiation. CX3CR1+ dendritic cells directly uptake antigens from the intestinal lumen and have greater inflammatory potential, whereas CD103+CX3CR1− dendritic cells have tolerogenic properties [12]. Goblet cell-associated antigen passages deliver antigens to CD103+CX3CR1− dendritic cells and, therefore, are associated with oral tolerance induction [13]. Migratory CD103+ dendritic cells from the lamina propria in the mesenteric lymph nodes can promote the development of gut-homing regulatory T cells through multiple mechanisms involving transforming growth factor-β (TGF-β), retinoic acid, transmembrane proteins, and enzymes involved in tryptophan catabolism [14-16].
Regulatory T cells, especially Foxp3+ regulatory T cells characterized by CD25 expression, play an essential role in oral tolerance. Foxp3 knockout mice developed multi-organ allergic inflammatory responses, whereas adoptive transfer of regulatory T cells could suppress anaphylaxis in a food allergy animal model [17,18]. Higher numbers of milk-specific CD4+CD25+ T regulatory cells were associated with the development of oral tolerance to cow’s milk [19,20]. Regulatory T cells can also produce several inhibitory cytokines, such as interleukin (IL)–10 and TGF-β. Interleukin (IL)-10 suppresses Th2 immune reactions and allergic inflammation by reducing IgE production and promoting allergen-specific IgG4 responses. Children who developed natural tolerance to hen’s eggs or peanuts had considerably increased IL-10 levels expressed by CD4+ T regulatory, CD25+ CD127lo, and Foxp3+ cells [21,22].
Immunosuppressive regulatory B cells regulate the immune responses by suppressing effector T cells via the production of suppressor cytokines such as IL-10 and TGF-β [23]. Also, IL-10-secreting regulatory B cells produce IgG4, a non-inflammatory isotype that prevents IgE-mediated mast cell and basophil degranulation [24].
Natural course of specific food allergies
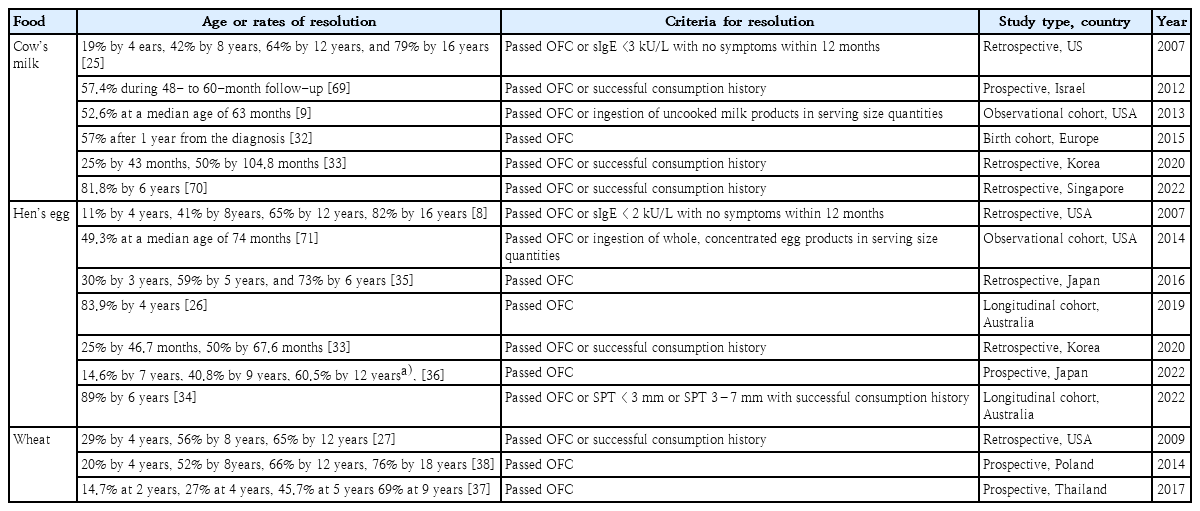
Numerous studies have shown that IgE-mediated allergies to cow’s milk, hen’s eggs, wheat, and soy are more likely to resolve in childhood, while allergies to peanuts, tree nuts, and seafood tend to persist [25-30]. However, the majority of studies on the natural course of food allergy were inherently limited by their design because population-based prospective studies using OFC at predetermined intervals are almost impossible to perform. Many past studies were retrospective analyses that included both OFC and medical history for assessing tolerance, whereas recent studies in Australia released longitudinal population-level challenge-based results of the natural course of food allergy in children [3,26,29]. The factors believed to affect the tolerance development or persistence of food allergy include symptom severity on ingestion, age at diagnosis, comorbid allergic diseases, and various immune-related markers that will be described later [31]. As the current status and results of studies related to the natural course of food allergy vary widely by the food in question, large-scale recent studies are mainly described in the text, while other various research results including Korean data for each major food allergy are listed in Tables 1 and 2 for the reference for each major food allergen.
1. Cow’s milk, hen’s egg, and wheat allergies
The EuroPrevall birth cohort study from 9 countries across Europe revealed that 56.5% of children with IgE-associated cow’s milk allergy passed double-blind placebo-controlled food challenges at re-evaluation 1 year after the initial diagnosis [32]. From a relatively large-scale retrospective analysis of children with cow’s milk allergy in the United States with a median follow-up duration of 54 months, the resolution rates of cow’s milk allergy were 19% by 4 years, 42% by 8 years, 64% by 12 years, and 79% by 16 years [25]. In a retrospective analysis of Korean children, half of the children with a cow’s milk allergy outgrew it at a median 8.7 years of age [33].
The resolution of challenge-proven hen’s egg allergy from a population-based prospective study in Australia was 89% by 6 years of age [34]. In Japan, where hen’s egg accounts for the highest frequency of all food allergies, the resolution rates were 30% by 3 years of age, 59% by 5 years of age, and 73% at 6 years of age in a retrospective analysis [35]. In a recent prospective report, a continuation of the previous study, the estimated acquired resolution rates were 14.6% by 7 years, 40.8% by 9 years, and 60.5% by 12 years [36]. In Korean children with immediate-type hen’s egg allergy, the median age at tolerance acquisition in 50% of patients was 5.6 years [33]. This result shows that the age of resolution of hen’s egg allergy is slightly younger than those reported in the United States and similar to that in Japan. A prospective larger-scale study is needed in Korea in the future.
In Thailand, where wheat allergy is particularly common and research is actively conducted, OFC was performed for evaluation of wheat allergy resolution if the patients’ specific IgE levels to wheat and ω-5 gliadin were ≤26 kUA/L and ≤1.06 kUA/L, respectively [37]. In this study, the proportion of children with wheat tolerance was 14.7% by 2 years, 27% by 4 years, 45.7% by 5 years, and 69% by 9 years. Another prospective analysis of 50 challenge-proven wheat-allergic children in Poland revealed that the resolution rates were 20% by 4 years, 52% by 8 years, 66% by 12 years, and 76% by 18 years [38].
As described above and summarized in Table 1, allergies to cow’s milk, hen’s eggs, and wheat are often outgrown before school age but not rarely persist into late childhood, so clinicians must pay attention to each individual patient when managing these allergies.
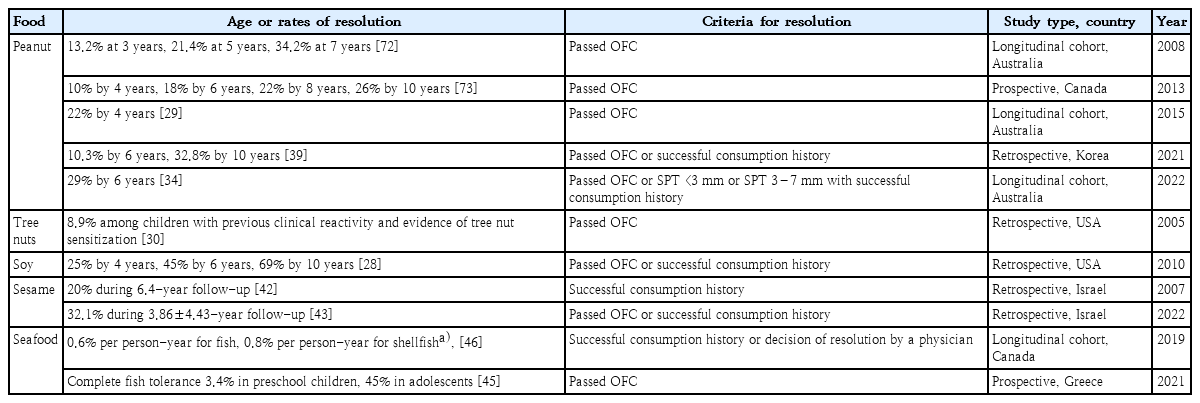
2. Legume, tree nut, and seed allergies
There are relatively few studies of the natural course of soy allergy compared to other food allergies. A retrospective analysis in a tertiary clinic in the United States reported that the resolution rates for soy allergy in children were 25% by age 4 years, 45% by age 6 years, and 69% by age 10 years [28]. Peanut allergy is a very active research field, especially in the West, and according to the latest Australian population-based prospective study, peanut allergy had resolved in 29% by 6 years of age [34]. As reported by the same research team, the rate of resolution of peanut allergy by the age of 4 years was reportedly 22% in 2015 [29]. A retrospective analysis conducted at 3 hospitals in Korea reported that the probabilities of peanut allergy resolution were 10.3% at age 6 years and 32.8% at age 10 years [39].
Tree nut allergy accounts for a considerable proportion of food allergies in children and frequently cause severe reactions, but the natural course of tree nut allergy compared to other major food allergies remains understudied [40,41]. A study published in 2005 that is still consistently cited when referring to the natural course of tree nuts reported a resolution rate of 8.9% among children with previous clinical reactivity and evidence of tree nut sensitization [30].
Most studies of sesame allergy have been published in Israel; according to a retrospective observation of 45 sesame allergy patients for 6 years, 20% achieved tolerance [42]. In a more recent retrospective analysis of 190 children with sesame allergy, 32.1 % had spontaneous resolution during the mean follow-up period of 3.86±4.43 years [43].
Among allergies to legumes, tree nuts, and seeds, only soy allergy seems to have good prognosis, but there are currently no Korean data of the natural course of soy allergy. Although walnuts are the most common cause of anaphylaxis in children aged 2–12 years in Korea, there is no study on the natural course of walnut allergy in Korean children, strongly suggesting the need for additional research in this field [44].
3. Seafood allergy
Studies of the natural course of seafood versus other allergies in children are relatively rare. A recently published prospective study examining the natural course of IgE-mediated fish allergy in Greek children reported a rate of 22% tolerance development to cod during the follow-up period [45]. Complete tolerance to fish increased with age from 3.4% in preschool children to 45% in adolescents in that study. According to a longitudinal cohort study of 49 children and 14 adults with seafood allergy in Canada, the resolution rate was 0.6% per person-year for fish and 0.8% per person-year for shellfish [46]. Reports on the natural course of shellfish allergy are rare, and no studies have been conducted in Asian children. In 11 adults with shrimp allergy, the levels of shrimp-specific IgE remained constant over a 24-month period suggesting the persistence of allergy [47].
Studies of the natural course of seafood allergy in Korean children have not been published to date. Contrary to the point that seafood allergy generally persists into adulthood, in actual clinical practice, it seems not uncommon for young children with fish allergy to improve within a few years after diagnosis, suggesting the need for future research into the natural course of seafood allergy in Korean children.
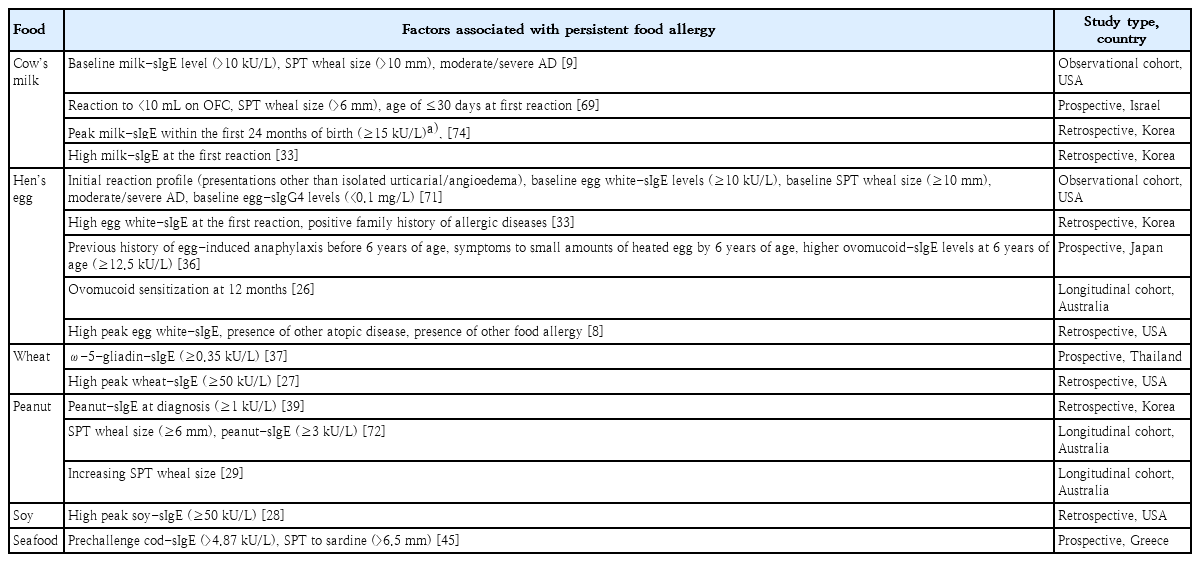
Factors associated with persistent food allergy
Evidence of markers predicting the resolution or persistency of food allergy remains insufficient today. Factors associated with the timing of food allergy resolution include age at diagnosis, comorbid allergic diseases and their severity, symptom severity on ingestion, skin prick test (SPT) reaction size, foodspecific IgE levels, rate of change of food-specific IgE levels or SPT reaction sizes, ratio of specific IgE to IgG4, diet, gut microbiome, and interventions such as oral immunotherapy (Fig. 1). The degree of sensitization to the specific food by serum-specific IgE levels or SPT is among few tools that can be repeatedly performed in the actual clinical setting to monitor the natural course of food allergy. Diagnostic cutoff levels for predicting the persistency of food allergy have been suggested by numerous studies, but the results varied widely by age, region, and study design bearing considerable limits when applied in clinical practice [48-51].
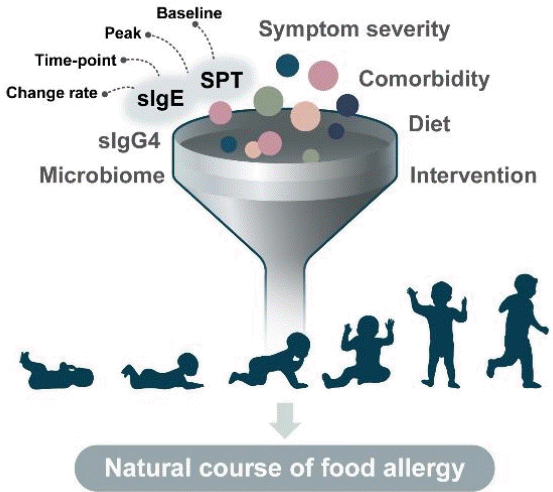
Suggested elements associated with the natural course of food allergies. sIgE, specific immunoglobulin E; SPT, skin prick test.
The extensive research results related to specific IgE for predicting OFC outcomes at specific time points are not covered in detail in this article, as they refer more to the diagnosis of food allergy. This review mainly examined and summarized the findings related to predicting persistent food allergy in studies that prospectively or retrospectively followed the natural course of food allergy over time (Table 3). Studies showed that the persistence of food allergy was considerably associated with the baseline, peak (highest at all ages), and/or time point food-specific IgE levels. The resolution rates of cow’s milk allergy by 10 years was 87% with a peak cow’s milk-specific IgE <2 kUA/L and 5% with a peak level of ≥50 kUA/L [25].
Component-resolved data in some foods may provide additional information to enable a more precise diagnosis as well as for persistence predictions of food allergy. High levels of ovomucoid- and casein-specific IgE were associated with persistent hen’s egg and cow’s milk allergies, respectively, but specific cutoff levels for ovomucoid- or casein-specific IgE for predicting persistent food allergy have not been reported to date [35,52]. High IgE levels to gliadins were correlated with persistent wheat allergy and the development of asthma in children [53]. Other components such as Ara h 2 and Ara h 6 from peanut, Jug r 1 from walnut, Cor a 9 and Cor a 14 from hazelnut, and Ana o 3 from cashew nut have been studied for their useful value in diagnosing food allergy, but the role of these components with regard to the natural course of food allergy should be further investigated [54-57].
Diet and the gut microbiome also influence the persistence of food allergy by influencing mucosal immune tolerance [58]. It was recently suggested that regular consumption of the food in the form of reduced allergenicity, such as baked products containing egg and/or milk, or at an amount that does not induce symptoms, may improve the prognosis of food allergy [59]. Certain strains such as Bifidobacterium longum and Bacteroides fragilis can induce intestinal regulatory T cells, possibly by pattern-recognition receptor activation on dendritic cells [60]. Metabolites such as short-chain fatty acids produced by bacteria after the digestion of dietary fibers play an important role in enhancing the regulatory activity of dendritic cells, leading to the induction of regulatory T cells and IL-10-secreting T cells [61].
Assessment of resolution of food allergy and possible interventions
It is imperative for clinicians to aptly guide the timing of food allergy resolution assessments using appropriate parameters. As the course of food allergy can vary substantially depending on food type, patient age, and test results, clinicians would have to integrate all available information to evaluate the patient’s food allergy resolution status. By incorporating the results of various studies (Table 3), Santos et al. recently suggested an approach to assessing food allergy resolution: for cow’s milk and hen’s egg, a baseline specific IgE <2 kUA/L, >50% decrease in specific IgE levels over >12 months, and specific IgE <3 kUA/L (cow’s milk) or <2 kUA/L (hen’s egg) at other time points were associated with likely resolution; for peanuts, a baseline specific IgE <2 kUA/L and other time point-specific IgE <2 kUA/L (if reaction) or <5 kUA/L (if no reaction) were associated with likely resolution; and for tree nuts, a baseline specific IgE <2 kUA/L was associated with likely resolution, whereas an SPT reaction size ≥13 mm and/or specific IgE ≥5 kUA/L at other time points were associated with likely persistence [62]. When considering OFC for the assessment of food allergy resolution, clinicians should consider multifaceted factors such as patent age, the importance of the food in the diet, previous history of reactions, potential for risk, comorbidities especially severe atopic dermatitis, uncontrolled asthma, or eosinophilic gastrointestinal diseases, and patient and family preferences (Fig. 2).
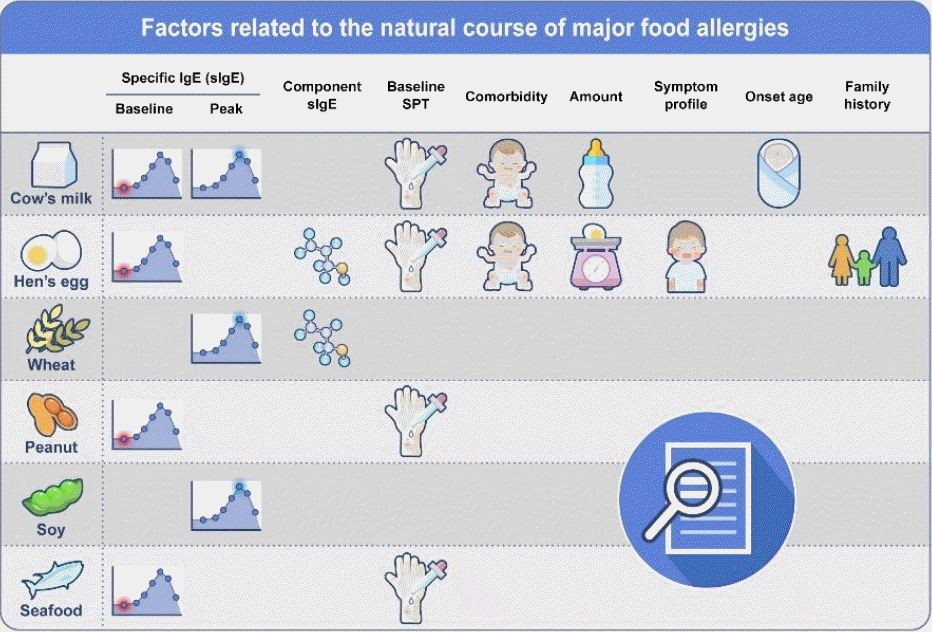
Factors related to the natural course of major food allergies. sIgE, specific immunoglobulin E; SPT, skin prick test.
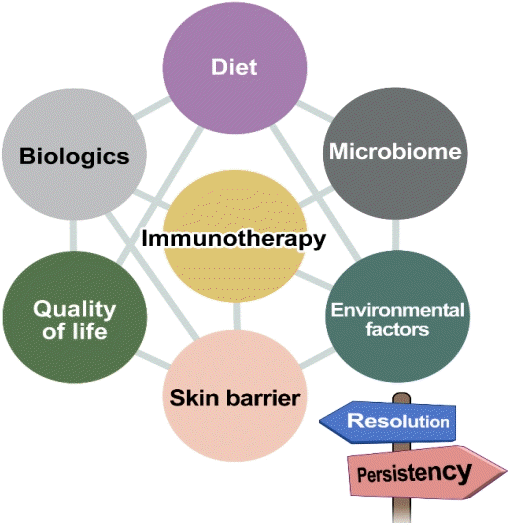
The current leading treatment for modifying the natural course of food allergy is oral immunotherapy, an allergen-specific approach based on progressive incremental ingestion of the food allergen doses until a daily maintenance dosage is reached to achieve desensitization. Although oral immunotherapy still requires some attention, such as adverse events occurring not rarely during the treatment and achievement of continued tolerance, its implementation is gradually expanding worldwide and studies proving its efficacy are abounding [63-65]. Epicutaneous immunotherapy has been actively studied, particularly for peanut allergy, and has shown safe and modest treatment responses, especially in younger children [66]. In cases of severe or multiple food allergies, studies of allergen-specific immunotherapy combined with biologics such as omalizumab have been reported, enabling faster dose escalation and lower rates of adverse reactions during immunotherapy [67,68]. The purpose of allergenspecific immunotherapy in food allergy may be sufficient tolerance induction in some patients or the increase of the tolerated dose to improve quality of life in others, which will not be discussed in detail here. Other than immunotherapy, aspects that may affect the natural course of food allergy are diet, gutmicrobial modulation, skin barrier management, psychological state, and other environmental factors, which are outlined in Fig. 3.
Conclusion
To provide the optimal treatment to patients with food allergy and help improve their quality of life, it is essential that clinicians understand the natural course of individual food allergies. At this point, it remains unclear why some children outgrow their food allergy and others do not. Past studies of the natural course of food allergy are heterogeneous and bear limitations in terms of study design, but lately, population-based prospective data are becoming available. Based on the various factors associated with food allergy persistence, health care practitioners should offer relevant information to patients and their caregivers and conduct relevant assessments to evaluate food allergy resolution at appropriate timing. Using therapeutic tools including allergen-specific immunotherapy, gut-microbial modulation, and other potential therapeutics to be developed, we may be able to alter the course of food allergy in the future.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.